
PATIENT MEDICATION INFORMATION
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE
Pr
TRULICITY
®
dulaglutide injection
For Subcutaneous Use Only
Read this carefully before you start taking Trulicity and each time you get a refill. This leaflet is a summary and
will not tell you everything about this drug. Talk to your healthcare professional about your medical condition
and treatment and ask if there is any new information about Trulicity.
Serious Warnings and Precautions
• In male and female rats, dulaglutide causes dose-dependent and treatment-duration-dependent thyroid
C-cell tumors (adenomas and carcinoma) after lifetime exposure. It is unknown whether Trulicity causes
thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans, as human relevance
could not be ruled out by clinical or nonclinical studies.
• Trulicity is contraindicated in patients with a personal or family history of MTC and in patients with
Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). It is unknown whether monitoring with serum
calcitonin or thyroid ultrasound will mitigate human risk of thyroid C-cell tumors. Patients should be
counseled regarding the risk and symptoms of thyroid tumors.
What is Trulicity used for?
Trulicity may improve blood sugar control in adults with type 2 diabetes mellitus in combination with:
• diet and exercise in patients for whom metformin is inappropriate due to contraindication or intolerance
• metformin, when diet and exercise plus maximal tolerated dose of metformin do not achieve adequate
blood sugar control
• metformin and a sulfonylurea, when diet and exercise plus dual therapy with metformin and a sulfonylurea
do not achieve adequate blood sugar control
• sodium glucose co-transporter 2 inhibitor (SGLT2i) with metformin, when diet and exercise plus SGLT2i
with or without metformin do not achieve adequate glycemic control
• basal insulin with metformin, when diet and exercise plus basal insulin with or without metformin, do not
achieve adequate blood sugar control
• mealtime insulin with metformin, when diet and exercise plus basal or basal-bolus insulin therapy (up to
two injections of basal or basal plus mealtime insulin per day) with or without oral diabetes medications, do
not achieve adequate blood sugar control
Trulicity may be used, along with diet and exercise, to reduce the risk of non-fatal stroke in adults with type 2
diabetes mellitus.
Trulicity is not a substitute for insulin. Trulicity should not be used in patients with type 1 diabetes mellitus
(formerly known as insulin-dependent diabetes mellitus or IDDM) or for the treatment of diabetic ketoacidosis
(a complication of diabetes with high blood sugar, rapid weight loss, nausea or vomiting).
Trulicity has not been approved in children under 18 years of age.
How does Trulicity work?
Trulicity belongs to a class of medicines called GLP-1 receptor agonists (glucagon-like peptide-1 receptor
agonists). Trulicity may lower blood sugar in adults with type 2 diabetes mellitus by helping your body release
more insulin when your blood sugar is high.

Trulicity
®
, dulaglutide Patient Medication Information Page 2 of 7
What are the ingredients in Trulicity?
Medicinal ingredients: dulaglutide
Non-medicinal ingredients: citric acid anhydrous, mannitol, polysorbate 80, trisodium citrate dihydrate
Trulicity comes in the following dosage forms:
Trulicity is a solution for injection. Trulicity is available as a single-use prefilled pen in either 0.75 mg/0.5 mL,
1.5 mg/0.5 mL, 3 mg/0.5 mL or 4.5 mg/0.5 mL strengths. Each pen contains one weekly dose of Trulicity.
Do not use Trulicity if:
• you are allergic to this drug or to any ingredient in the formulation or component of the container.
• you or a member of your family has ever had medullary thyroid cancer.
• you have Multiple Endocrine Neoplasia syndrome type 2 (MEN 2)
• you are pregnant or breastfeeding.
To help avoid side effects and ensure proper use, talk to your healthcare professional before you take
Trulicity. Talk about any health conditions or problems you may have, including if you:
• or a member of your family has or has had medullary thyroid carcinoma, or if you have Multiple Endocrine
Neoplasia syndrome type 2 (MEN 2).
• have type 1 diabetes.
• have ever had diabetic ketoacidosis (increased ketones in the blood or urine).
• have ever had an allergic reaction to Trulicity
• are taking an anti-diabetic medicine known as a sulfonylurea (e.g., glyburide, gliclazide, glimepiride) or
insulin. Your doctor may want to reduce your dose of sulfonylurea or insulin when you take it together with
Trulicity in order to avoid low blood sugar. Take precautions to avoid low blood sugar while driving or using
machinery.
• have or have had pancreas problems such as inflammation of the pancreas.
• have severe problems with your stomach (gastroparesis) or food digestion. Trulicity slows stomach
emptying so food passes more slowly through your stomach.
• are pregnant or plan to become pregnant.
• are breastfeeding or plan to breastfeed.
• have a high heart rate (fast pulse).
• have a condition called heart block.
• have any heart disease, such as angina, heart rhythm disturbances or congestive heart failure; or if you
have ever had a myocardial infarction (heart attack).
• have kidney problems.
• have liver problems.
• have severe vomiting and/or diarrhea and/or dehydration.
Other warnings you should know about:
• See “Serious Warnings and Precautions” black box.
• Heart rate increase and PR interval prolongation. Trulicity may increase heart rate and could cause
changes known as PR prolongation, which are detected by electrocardiogram (ECG) tracings. Increased
heart rate is the same as a faster pulse. Rarely, drugs with these effects can cause changes in heart
rhythm that could result in dizziness, palpitations (a feeling of rapid, pounding, or irregular heart beat),
fainting or death. These heart rhythm changes are more likely if you have heart disease, or if you are taking

Trulicity
®
, dulaglutide Patient Medication Information Page 3 of 7
certain other drugs. It is important to follow your doctor's advice about the dose of Trulicity or about any
special tests that you may need.
• Inflammation of your pancreas (pancreatitis). Stop using Trulicity and call your healthcare provider right
away if you have severe pain in your stomach area (abdomen) that will not go away, with or without
vomiting. You may feel pain from your abdomen to your back. It is not known if Trulicity can be used in
people who have had pancreatitis.
• Gastrointestinal disorders. Trulicity is not recommended for use in people with severe stomach or intestinal
problems. Stomach problems, sometimes severe, have been reported in people who use Trulicity. Tell your
healthcare provider if you have stomach problems that are severe or will not go away.
• Food or liquid getting into lungs during anesthesia. Some patients taking medicines like Trulicity have had
problems with food or liquid from their stomach getting into their lungs while under general anesthesia or
deep sedation. Tell your healthcare provider that you are taking Trulicity before you have a procedure that
requires general anesthesia or deep sedation.
• Low blood sugar (hypoglycemia). Your risk for getting low blood sugar may be higher if you use Trulicity
with another medicine that can cause low blood sugar, such as a sulfonylurea or insulin.
• Serious allergic reactions. Stop using Trulicity and get medical help right away if you have any symptoms
of a serious allergic reaction including itching, rash, or difficulty breathing.
• Kidney problems (kidney failure). In people who have kidney problems, diarrhea, nausea, and vomiting
may cause a loss of fluids (dehydration) which may cause kidney problems to get worse.
• Dehydration: Nausea, vomiting and diarrhea can lead to dehydration. It is important to avoid dehydration
which can cause serious kidney problems even in people with normal kidney function.
Talk to your healthcare provider about any side effect that bothers you or does not go away. These are not all
the possible side effects of Trulicity.
Trulicity is not approved for use in children under 18 years of age.
Tell your healthcare professional about all the medicines you take, including any drugs, vitamins,
minerals, natural supplements or alternative medicines.
The following may interact with Trulicity:
• A sulfonylurea medicine (e.g., glibenclamide or glimepiride) or insulin. This is because using Trulicity at the
same time may cause your blood sugar to get too low (hypoglycemia). When you first start using these
medications together, your doctor may tell you to lower the dose of the sulfonylurea or insulin.
The following list includes some, but not all, of the drugs that may increase the risk of heart rhythm problems
while receiving Trulicity. You should check with your doctor or pharmacist before taking any other medication
with Trulicity:
• Drugs to treat hypertension.
• Drugs to treat heart failure.
• Drugs to treat HIV infection.
• Drugs to treat attention deficit-hyperactivity disorder.
• Drugs to suppress appetite/cause weight loss.
• Decongestants.
• Drugs to treat asthma.
Tell your healthcare professional about all the medicines you take, including any drugs, vitamins, minerals,
natural supplements or alternative medicines.

Trulicity
®
, dulaglutide Patient Medication Information Page 4 of 7
How to take Trulicity:
• Before using Trulicity, talk to your doctor about low blood sugar and how to manage it.
• Take Trulicity exactly as your physician has prescribed.
• Read the Instructions for Use leaflet for instructions on how to use the Trulicity pen.
• Talk to your healthcare provider about how to correctly administer Trulicity before you use it for the first
time. If you do not understand the instructions or have any questions, talk with your doctor, diabetes nurse,
or pharmacist.
• Trulicity is an injection which is given under the skin (subcutaneously). The Trulicity injection pen has been
shown to be easy to learn and easy to use. Do not inject Trulicity into a vein or muscle. The best places to
give yourself the injection are your stomach area (abdomen), upper leg (thigh), or upper arm. Do not use
the same site for each injection. Change (rotate) your injection site with each weekly injection.
• You can give yourself the injection at any time of the day.
• If you give yourself insulin in addition to Trulicity, never mix them in the same container. Give yourself
separate injections of insulin and Trulicity. You may give both injections in the same body area (for
example, your stomach area), but not right next to each other.
• Do not share your pen, or needles with another person. You may give another person an infection or get an
infection from them.
• Keep pens and needles out of the reach of children.
Usual dose:
The recommended starting adult dose is 0.75 mg once weekly administered subcutaneously (under the skin).
The dose may be increased to 1.5 mg once-weekly based on your blood sugar response. After at least four
weeks your doctor may increase your dose to 3 mg once weekly if your blood sugar is not well controlled on
the 1.5 mg dose. After at least four weeks your doctor may further increase your dose to 4.5 mg once weekly if
your blood sugar is not well controlled on the 3 mg dose. The maximum recommended dose is 4.5 mg once-
weekly.
Trulicity can be taken any time of the day, with or without food.
Use Trulicity exactly as prescribed. Do not change your dose or stop Trulicity without talking to your doctor.
Your doctor should start you on a diet and exercise program when you start taking Trulicity. Stay on this
program while you are taking Trulicity. The response on your blood sugar control should be monitored by
periodic measurements of blood glucose and HbA1c levels.
Your dose of Trulicity and other diabetes medicines may need to change because of change in level of
physical activity or exercise, weight gain or loss, increased stress, illness, change in diet, or because of other
medicines you take. Talk to your doctor to seek medical advice promptly.
Overdose:
If you think you, or a person you are caring for, have taken too much Trulicity, contact a
healthcare professional, hospital emergency department, or regional poison control centre
immediately, even if there are no symptoms.
Missed Dose:
If you miss a dose of Trulicity, take your missed dose as soon as possible if there are at least 3 days (72
hours) until your next scheduled dose. If there are less than 3 days remaining, skip the missed dose and take
your next dose on the regularly scheduled day. Do not take 2 doses of Trulicity within 3 days of each other.
The dosing day of your weekly administration can be changed if necessary, as long as there are at least 3
days between doses.
Trulicity
®
, dulaglutide Patient Medication Information Page 5 of 7
What are possible side effects from using Trulicity?
These are not all the possible side effects you may have when taking Trulicity. If you experience any side
effects not listed here, tell your healthcare professional.
Very Common (≥1 in 10):
Nausea
Diarrhea
Vomiting
Abdominal pain
Low blood sugar (hypoglycemia) when used in combination with other diabetes medicines especially
metformin, insulin, or secretagogues (e.g. sulfonylurea)
If nausea happens, it is most common when first starting Trulicity. In most people, nausea decreases over time
as their body gets use to the medicine.
Common (≥1 in 100 and <1 in 10):
decreased appetite
upset stomach (dyspepsia)
constipation
gassiness (flatulence)
abdominal distension
heartburn (gastroesophageal reflux disease)
belching (eructation)
fatigue
fast heartbeat (sinus tachycardia)
first degree atrioventricular block (AV block)
hypoglycemia when used as monotherapy and in combination with metformin and pioglitazone
Uncommon (≥1 in 1000 and <1 in 100):
injection site reaction
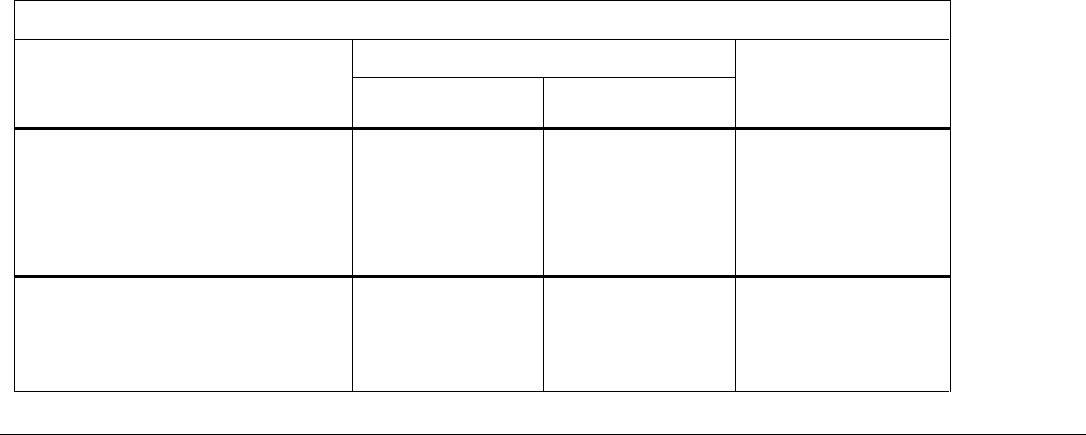
Serious side effects and what to do about them
Symptom / effect
Talk to your healthcare professional
Stop taking drug
and get immediate
medical help
Only if severe
In all cases
UNCOMMON
Severe hypoglycemia* (low
blood sugar) symptoms:
disorientation, loss of
consciousness, or seizures
✓
Thyroid tumour symptoms: lump
in the neck, difficulty in
swallowing difficulty in breathing
or persistent hoarseness
✓

Trulicity
®
, dulaglutide Patient Medication Information Page 6 of 7
Atrial fibrillation/ flutter, irregular
heart rate, palpitations, fatigue
or shortness of breath
✓
✓
RARE
Severe allergic reaction
(anaphylactic reaction)
symptoms: breathing problems,
swelling of throat and face, and
fast heartbeat.
✓
✓
Pancreatitis symptoms:
prolonged severe abdominal
pain with or without vomiting
✓
✓
*The risk of severe hypoglycemia is dependent on the other medications you may be taking.
If you have a troublesome symptom or side effect that is not listed here or becomes bad enough to interfere
with your daily activities, tell your healthcare professional.
Reporting Side Effects
You can report any suspected side effects associated with the use of health products to
Health Canada by:
• Visiting the Web page on Adverse Reaction Reporting
(https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-
canada.html) for information on how to report online, by mail or by fax; or
• Calling toll-free at 1-866-234-2345.
NOTE: Contact your healthcare professional if you need information about how to manage
your side effects. The Canada Vigilance Program does not provide medical advice.
Storage:
• Trulicity should be stored in the refrigerator at 2C to 8C, up to the expiration date. Do not use Trulicity
beyond the expiration date.
• Do not freeze. Do not use Trulicity if it has been frozen.
• Do not store in the freezer.
• Protect from light.
• Each single-use prefilled pen may be stored unrefrigerated for up to 14 days at a temperature not to
exceed 30ºC.
• The Trulicity prefilled pen must be discarded after use in a puncture-resistant container.
Keep out of reach and sight of children.
If you want more information about Trulicity:
• Talk to your healthcare professional
• Find the full product monograph that is prepared for healthcare professionals and includes this Patient
Medication Information by visiting the Health Canada website: (https://www.canada.ca/en/health-

Trulicity
®
, dulaglutide Patient Medication Information Page 7 of 7
canada/services/drugs-health-products/drug-products/drug-product-database.html; the manufacturer’s
website www.lilly.ca, or by calling 1-888-545-5972.
This leaflet was prepared by Eli Lilly Canada Inc.
Last Revised: July 05, 2024
Trulicity is a registered trademark owned by or licensed to Eli Lilly and Company, its subsidiaries or affiliates.
TRU-0010-CA-PMI-20240705
