
Human Research Protection Program
Yale Research
Community Training
New Yale HRPP/IRB Manuals &
Related Documents
Yale University
Human Research Protection Program
January 2023
1

Human Research Protection Program
TRAINING OVERVIEW
2

Human Research Protection Program
Training Overview
• The Yale Human Research Protection Program (HRPP) is excited
to announce the upcoming launch of the new Yale HRPP Policy
and Standard Operating Procedure Manual, updated
Investigator Manual, and updated submission forms and
related documents.
• The effective date for these documents is January 30, 2023.
• This session will provide a high-level overview of these
documents, including a discussion regarding significant new or
updated information found in the documents that the Yale
research community should be aware of.
3

Human Research Protection Program
Learning Objectives
• Understand the purpose, structure, and general content of all new
and/or updated HRPP documents.
• Obtain a foundational understanding of new and/or updated
regulatory information and Yale HRPP/IRB processes that may
impact study teams and the research community broadly.
• Understand where to access all relevant documents.
4
This Photo by Unknown Author is licensed under CC BY-SA

Human Research Protection Program
YALE HRPP POLICY AND STANDARD
OPERATING PROCEDURE MANUAL
Yale Research Community Training
6

Human Research Protection Program
Yale HRPP Policy and Standard Operating Procedure
Manual – Overview
• This purpose of the manual is to consolidate existing standalone
Yale HRPP policies, procedures, and select guidance documents
into one streamlined manual.
• The manual also includes new topics related to human subjects
research conduct and oversight, reflecting the most up-to-date
regulatory information.
• Located in the IRES IRB Library (Handbooks and Manuals tab).
7
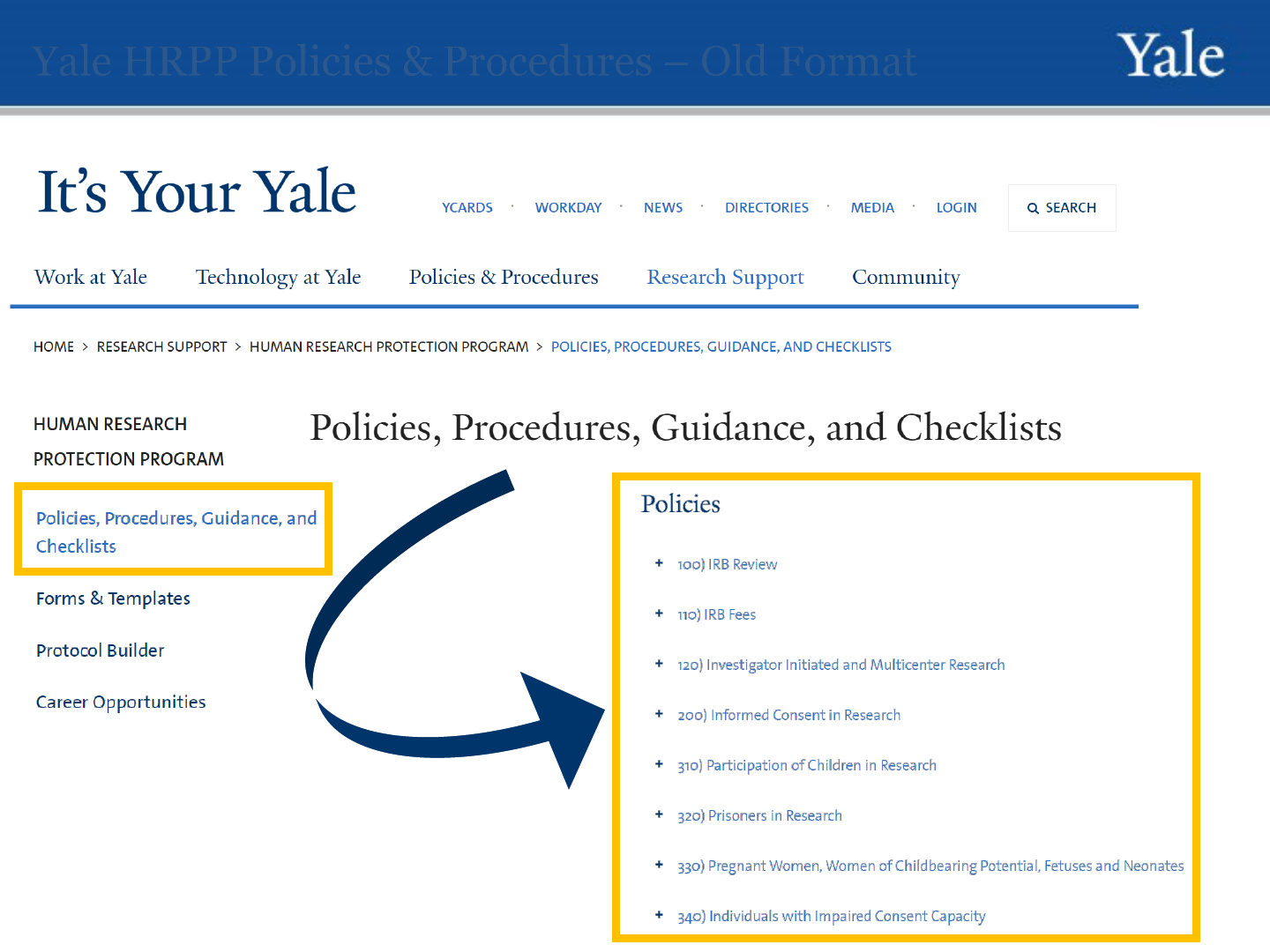
Human Research Protection Program
Yale HRPP Policies & Procedures – Old Format
8

Human Research Protection Program
Yale HRPP Policies & Procedures – Old Format
9
Example: Informed Consent in Research

Human Research Protection Program
Yale HRPP Policy and Standard Operating Procedure
Manual – New Format
10
• Most Yale HRPP policies,
procedures, and guidance
documents have been
consolidated into the new Yale
HRPP Policy and Standard
Operating Procedure Manual.
• The manual is divided into two
(2) parts:
Part I outlines Yale HRPP
policies and procedures.
Part II encompasses standalone
policies, procedures, and
guidelines that could not be
consolidated into Part I for a
variety of reasons.

Human Research Protection Program
Yale HRPP Policy and Standard Operating Procedure
Manual – Layout
11
Sections
Sub- Sections
Each section
and sub-
section is
hyperlinked to
the appropriate
page of the
PDF for easy
navigation.

Human Research Protection Program
Yale HRPP Policy and Standard Operating Procedure
Manual – Search Function
12
You can also use the “Ctrl” + “F” function to search for specifics topics/words/phrases

Human Research Protection Program
Yale HRPP Policy and Standard Operating Procedure
Manual – Part I
13
• Part I of the Yale HRPP Policy and
Standard Operating Procedure Manual
covers everything from the mission of
the Yale HRPP, IRB functions,
investigator responsibilities, informed
consent requirements, reporting
requirements, considerations for
enrolling vulnerable populations,
requirements for federally funded
research studies, and many other
related research topics.
• This manual is the “go to” document
for referencing federal, state, and Yale
HRPP policies, procedures, and
guidance information related to
human research protections and
research requirements.

Human Research Protection Program
Yale HRPP Policy and Standard Operating Procedure
Manual – Part I
14
Yale HRPP Policy & Standard Operating Procedure Manual Sections (Part I)
1 Human Research Protection Program 25 Conflict of Interest in Research
2 Quality Assurance 26 Participant Outreach
3 Education & Training 27 Health Insurance Portability and Accountability Act (HIPAA)
4 “Human Subjects” and “Research” Determinations 28 IRB and HRPP Billing
5 Exempt Determinations 29 Select State Statues Related to Human Subjects Research
6 IRB Reliance 30 ICH-GCP E6
7 Research Previously Approved by Another IRB 31 Databases, Registries, & Repositories
8 HRPP Business Continuity 32 Research Involving or Generating Genetic Information
9 Yale Institutional Review Board 33 Incidental Findings with Possible Health and Safety Significance for
Research Participants
10 IRB Actions, Failure to Respond, Appeals 34 NIH Data Management and Sharing
11 IRB Review Process 35 Genomic Data Sharing (GDS)
12 Suspensions, Terminations, and Investigator Holds 36 Certificates of Confidentiality
13 Documentation and Records 37 Self-Experimentation
14 Obtaining Informed Consent from Research Subjects 38 Student Research
15 Vulnerable Subjects in Research 39 Community Based Research
16 FDA-Regulated Research 40 Transnational Research
17 Unanticipated Problems Involving Risks to Subjects or Others 41 Lead Investigator/Coordinating Center
18 Noncompliance 42 Research Involving the VA Connecticut Healthcare System
19 Complaints 43 Department of Defense
20 Other Reportable Information 44 Department of Justice
21 Reporting to Federal Agencies, Departments, and Organizational Officials 45 Department of Energy
22 Reporting to AAHRPP 46 Environmental Protection Agency
23 Investigator Responsibilities 47 Department of Education
24 Sponsored Research

Human Research Protection Program
Yale HRPP Policy and Standard Operating Procedure
Manual – Part I: NEW or REVISED Content
15
New Content or Areas of Heavily Revised Content/Layout
• Section 6 – IRB Reliance
• Section 8 – HRPP Business Continuity
• Section 10 – IRB Actions, Failure to Respond, Appeals
• Section 37 – Self-Experimentation
• Section 39 – Community Based Research
Streamlined Navigation for Study Team Regarding Reporting
Requirements/Guidance (content has not changed)
•
Section 12 – Suspensions, Terminations, and Investigator Holds
•
Section 17 – Unanticipated Problems Involving Risks to Subjects or Others
•
Section 18 – Noncompliance
•
Section 19 – Complaints
•
Section 20 – Other Reportable Information

Human Research Protection Program
Yale HRPP Policy and Standard Operating Procedure
Manual – Part II
16
• Part II of the of the Yale HRPP Policy
and Standard Operating Procedure
Manual contains links to additional
standalone HRPP policies,
procedures, and guidelines relevant
to the conduct of human subjects
research under the purview of the
Yale HRPP (Section A).
• Additional relevant Yale policies,
procedures, and guidelines under
the authority of other University
departments, offices, or programs
reside elsewhere. However, links to
key policies, procedures, and
guidelines are included in Part II
(Section B)

Human Research Protection Program
Yale HRPP Policy and Standard Operating Procedure
Manual – Part II
17
Yale HRPP Policy & Procedure Manual Sections (Part II)
Section A: Yale Human Research Protection Program Standalone Policies, Procedures, and Guidelines
HRPP Policy on Oversight of Yale Sponsor-Investigator held Investigational New Drug (IND) Applications, Investigational Device Exemptions
(IDE) or Emergency Use or Emergency Use Authorizations (EUA)
HRPP Policy on Institutional Conflicts of Interest In Human Research
Research Recruitment at Yale and Yale New Haven Hospital
Guidance on Committee Reviews Required for Human Subjects Research Protocols Using Radiation
Clinical Trial Registration and Reporting Requirements
Guideline - Quality Improvement Projects (Clinical Setting)
Yale Human Research Protection Program Business Continuity Plan
Section B: Yale University and Other University Schools, Departments, Offices, or Programs Standalone Policies, Procedures,
and Guidelines
University Policy 1360, Human Research Protection
University Policies & Procedures
Researchers: Policy guidelines for Yale Faculty, Students, and Staff governing Research at Yale
Research Administration University-Wide Policy Documents
Yale Research Support
Yale Faculty Handbook
Yale University Policy on Conflict of Interest

Human Research Protection Program
UPDATED INVESTIGATOR MANUAL
Yale Research Community Training
18

Human Research Protection Program
Investigator Manual – Overview of Changes
• Added new sections:
Working with External IRBs;
Instructions on requesting Yale IRB to serve as the sIRB for a multi-site research
project;
Data Sharing Plans for federal grant applications; and
Description of ancillary committees.
• Revised format – sections were streamlined (topics grouped accordingly) and
the question-and-answer format was removed.
• Updated the information regarding regulatory requirements for research
subject to federal agencies’ oversight.
• Added instructions that previously lived as standalone documents (e.g.,
obtaining Certificates of Confidentiality, adding Unaffiliated Investigators,
submitting RNI vs. MODs, etc.).
19

Human Research Protection Program
Investigator Manual – Format
20
Old format
Revised format

Human Research Protection Program
Investigator Manual – How to Use this Resource
• Think about the Investigator Manual as the “how-to” manual for
investigators and research staff. The information contained in the document
may need to be supplemented with additional regulations and agency
guidance.
• If a question arises during the conduct of research, look for guidance in the
Investigator Manual first. You may also review the HRPP Policy and Standard
Operating Procedure Manual to understand any relevant regulatory
frameworks.
– For example, the HRPP Policy and Standard Operating Procedure Manual will include
information about Certificates of Confidentiality with instructions for the IRB
regarding when one should be required, while the Investigator Manual will include
instructions for the investigator on how to obtain one.
• Located in the IRES IRB Library (Handbooks and Manuals tab) - Same
location as the HRPP Policy and Standard Operating Procedure Manual.
21

Human Research Protection Program
UPDATED CHECKLISTS,
WORKSHEETS, AND OTHER FORMS
Yale Research Community Training
22

Human Research Protection Program
Yale HRPP/IRB Checklists – Summary and Location
Checklists are used by the IRB reviewers to document required IRB determinations.
Investigators may consult the checklists to understand the regulatory requirements related
to research – however Investigators are not required to complete the checklists. They will
continue to be posted in the Checklists tab of the IRES IRB Library.
23

Human Research Protection Program
Yale HRPP/IRB Checklists – Changes to Documents
The following checklists were recently revised for clarity or new regulatory
requirements. The effective date for these checklists is January 30, 2023:
• HRP-414 – CHECKLIST – Neonates of Uncertain Viability
• HRP-412 – CHECKLIST – Pregnant Women
• HRP-411 – CHECKLIST – Waiver of Written Documentation of Consent
• HRP-417 – CHECKLIST – Cognitively Impaired Adults
PLEASE NOTE: The revisions made to the checklists are not substantial and will
not impact study teams or the conduct of research. The changes have been
made to better assist Yale IRB staff with reviewing research applications
involving the above topics.
24

Human Research Protection Program
Yale HRPP/IRB Worksheets – Summary and Location
Worksheets provide the IRB reviewers with additional information about regulatory
requirements and guidance related to the IRB review and approval of research. Investigators
may consult the worksheets to understand the regulatory landscape related to human
subjects research - however Investigators are not required to complete the worksheets. They
will continue to be posted in the Worksheets tab of the IRES IRB Library.
25

Human Research Protection Program
Yale HRPP/IRB Worksheets – Changes to Documents
The following worksheets were revised for clarity or new regulatory requirements.
The effective date for these worksheets is January 30, 2023:
• HRP-332 – WORKSHEET – NIH GDS Institutional Certification
• HRP-307 – WORKSHEET – Devices
• HRP-311 – WORKSHEET – Engagement Determination
• HRP-331 – WORKSHEET – FERPA Compliance
• HRP-306 – WORKSHEET – Drugs and Biologics
• HRP-312 – WORKSHEET – Exemption Determination
• HRP-317 – WORKSHEET – Short Form of Consent Documentation
• HRP-318 – WORKSHEET – Additional Federal Criteria
• IRB Member Review Worksheet – Initial
PLEASE NOTE: Most revisions made to the worksheets are not substantial and will
not impact study teams or the conduct of research. The biggest change was the
revision of HRP-312 (Exemption Determination), which was updated to reflect the
updated 2018 Common Rule exemption categories.
26

Human Research Protection Program
Yale IRB Submission Form – Overview of Changes
Two types of changes:
• Addition of new sections (required only for certain types of studies).
• Addition of new questions/reminders to existing sections.
What the changes mean for the research community:
• Use of the revised submission form is only applicable to new submissions of initial
studies.
• Initial submissions of protocols using older version of the form will be rejected as of
March 1, 2023.
• The IRB may request that the investigator add any of the applicable new
sections/questions to the existing IRB Submission Form at the time of a specific
modification (e.g., submission of modification to add a new federal grant that includes a
data sharing plan may mean that the new supplement on data sharing be added to the
existing IRB Submission Form*)
*This is consistent with the current practice (e.g., Supplement on Multicenter Management must be
completed at the time of a modification to add new sites to the protocol and where the Yale site becomes
the coordinating center).
27

Human Research Protection Program
Yale IRB Submission Form – New Sections
Section Description
Supplement V: Research
with Tobacco Products
Complete only when the research includes the
use of tobacco products.
Supplement IX: Research
Under an IND or IDE Held by
a Yale Investigator
Complete only if the research involves use of a
drug conducted under an IND held by a Yale
investigator or a device study conducted under an
IDE held by the Yale investigator; currently this
supplement exists as a stand-alone document;
the information is used by the YCCI IND/IDE
Management Office.
Supplement X: Ancillary
Committees
Investigators should review this section to
identify any ancillary committees applicable to
the research.
Supplement XI: Data Sharing
and Management Policy
Complete if the study is subject to the NIH Data
Sharing and Management Policy.
28

Human Research Protection Program
Yale IRB Submission Form – Supplement V – Tobacco
Products
29
The supplement is
only for studies that
involve tobacco
products as the
Drugs page in IRES
IRB does not
include specific
questions relevant
to tobacco products

Human Research Protection Program
Yale IRB Submission Form – Supplement X - Ancillary
Committees
30
Instructions on how and
when to obtain approval
from the committee
Name of the ancillary
committee
Description of
when the
ancillary review
applies; if the
scenario applies
to your research,
check off the box
in the second
column

Human Research Protection Program
Yale IRB Submission Form – Supplement XI – Data
Management and Sharing
31
Applies to federally
funded studies with the
grant application dated
January 25, 2023 or later;
additional information is
available in the
Investigator Manual and
on the OSP website:
https://tst.your.yale.edu/r
esearch-support/office-
sponsored-projects/data-
management-and-
sharing-dms

Human Research Protection Program
Yale IRB Submission Form – Revised Sections
Section Description
Section 4.
Confidentiality and
Privacy
Questions added regarding:
• Cloud data storage
• Data Use Agreements
• Material Transfer Agreements
• Privacy of research participants
Guidance added regarding:
• Business Associate Agreements
• Data classification
SUPPLEMENT VIII:
Special Populations
Question added regarding:
• The consent process for non-English speaking individuals to
ensure the process is conducted in the language understood
by the potential participant
Guidance added regarding:
• FDA expectations regarding translation of the consent
document if a short form is used for enrollment
32

Human Research Protection Program
Request to Use External IRB Form – Overview of
Changes
• Form that must be completed at the time of the initial request to use an
external IRB (with the exception of NCI CIRB studies).
• Will be required for submissions on January 30, 2023.
• Must be uploaded as the category ‘Request to Use External IRB’ in the
Local Site Documents page (Other attachments [Item #3]).
33

Human Research Protection Program
Request to Use External IRB Form – Summary
• The Request to Use External IRB form asks questions relevant to
the institutional review of local context:
• Recruitment of participants
• Storage of data
• HRPP billing instructions
• Data Use Agreements and Material Transfer Agreements
• Review by Ancillary Committees
• Research under an IND or IDE held by a Yale Investigator
• Data Management and Sharing for NIH funded studies
• Most of the questions and guidance are identical to the IRB
Submission Form (used for initial submission to Yale IRB).
34

Human Research Protection Program
COA Form for Billable Protocol Charge Information –
Overview
• This is a new document that must be submitted for all CRs and MODs/MODCRs
for billable protocols for which the study specific COA (chart of account) has not
been provided.*
• The COA form must be uploaded in IRES IRB as follows:
– For CRs and MOD/CRs, upload in the “Attach supporting documents” section of the
“Continuing Review/ Study Closure Information” page of the application.
– For MODs, upload in the “Other attachments” section of the “Local Site Documents”
page of the application.
• For information about the billing process, see the HRPP announcement dated
July 12, 2022, on the HRPP website
.
*IRB Submission Form includes charging instructions for invoicing the IRB review fees,
however, when the study specific COA is not available at the time of the initial review, the
investigator must provide a general departmental account.
35

Human Research Protection Program
Location of New/Revised Forms
36
Location of IRB Submission Form, Request
to Use External IRB Form, and COA Form

Human Research Protection Program
Training Summary
• The Yale HRPP has streamlined our policies, procedures, guidance documents,
worksheets, checklists, and other related documents and we have reimagined
some of the ways in which we educate our research community and HRPP
team.
• The majority of Yale HRPP/IRB policies and processes are not changing. The
manuals are simply new ways of providing information to our research
community, with some minor regulatory changes included.
• The effective date for all new/revised documents is January 30, 2023.
• All new/revised documents will be posted in the IRES IRB Library.
• The Yale HRPP is committed to a partnership with the research community in
the promotion of safe and ethical research. Yale HRPP/IRB staff are available to
the research community for consultation, education, and guidance.
37

Human Research Protection Program
Reference: HRPP and IRB Leadership
38
HRPP Main
Office Number
and Helpline
Access:
25 Science Park, 150 Munson St., 3
rd
Floor 203-785-4688 or
HRPP
Leadership
Linda Coleman, Director, HRPP
Jessica Rowe, Associate Director, HRPP/YCCI
Monika Lau, Assistant Director, HRPP & Deputy
HIPAA Privacy Office, HIPAA Data Privacy Office
Cathi Montano, Assistant Director, HRPP
Matt Stafford, Assistant Director, HRPP
Alan Teller, Assistant Director, HRPP
Michele Antisdel, Manager, HRPP - Institutional
Review
Brandy Lagner, Manager, HRPP - Operations
Gina Larsen, Senior Manager, HRPP
Amanda Liss, Manager, HRPP - Yale IRBs
Dawn Pedevillano, Senior Manager, HRPP -
Yale IRBs
Meriam Worzella, Manager, HRPP
linda.coleman@yale.edu
jessica.rowe@yale.edu
monika.lau@yale.edu
cathleen.montano@yale.edu
m.stafford@yale.edu
alan.teller@yale.edu
michele.antisdel@yale.edu
brandy.lagner@yale.edu
gina.larsen@yale.edu
amanda.liss@yale.edu
dawn.pedevillano@yale.edu

Human Research Protection Program
Questions?
Thank you for your dedication to
protecting research participants and for
conducting ethical research at Yale!
39
Please Document Your
Attendance Here:

