
January 27, 2020
Sen. Gene Suellentrop
Chair, Public Health and Welfare Committee
Capitol Building, Room 441-E
Topeka, Kansas 66612
Rep. Brenda Landwehr
Chair, Health and Human Services Committee
Capitol Building, Room 352-S
Topeka, Kansas 66612
RE: Report on Kansas Prescription Drug Monitoring Program (K-TRACS)
Dear Committee Chairs:
Pursuant to K.S.A. 65-1691, the Kansas State Board of Pharmacy is required to submit to the Senate Committee on Public
Health and Welfare and the House Committee on Health and Human Services a report on the Kansas Prescription Drug
Monitoring Program (PDMP) which tracks and monitors Schedule II through IV controlled substances and other drugs of
concern in Kansas. The Kansas PDMP, called K-TRACS, is a potent tool in aiding in the identification of patients with
drug-seeking behaviors, providing treatment, and educating the public. Each dispenser (pharmacy) is required to
electronically submit information to K-TRACS for each controlled substance prescription or drug of concern dispensed in
an outpatient setting. The goal of the PDMP is to prevent the misuse, abuse, and diversion of controlled substances and
drugs of concern while ensuring continued availability of these medications for legitimate medical use.
K-TRACS includes all retail and outpatient dispensing records for any controlled substance or drug of concern dispensed
in Kansas or to a Kansas resident, regardless of whether the pharmacy is located in Kansas. The only exception is for
quantities dispensed in the emergency room for 48 hours or less. If a prescriber or a pharmacist has a concern about a
patient, he/she can look up the patient’s prescription history in K-TRACS. Because K-TRACS is a real-time, web-based
system, patient information can be obtained instantly from any location at any time with the proper login credentials.
Prescribers and pharmacists must register for K-TRACS through the Board prior to utilizing the system. Law enforcement
and other state agencies have limited access to the program but may request records with proper legal authority. In
addition, de-identified or aggregate data may be provided to requestors for educational or research purposes. The Board
collaborates with the Kansas Department of Health and Environment (KDHE) to transmit such de-identified data and
receive reports and analysis based on KDHE’s research.
Advisory Committee:
The Act also created a PDMP Advisory Committee, subject to the oversight of the Board, composed of prescribers and
dispensers from various healthcare disciplines. In 2012, the Committee was authorized to review and analyze data for
purposes of identifying patterns and activity of concern; notify prescribers and dispensers who prescribed or dispensed the
prescriptions; notify law enforcement or appropriate regulatory boards for additional investigation; and utilize volunteer
peer review committees of professionals with expertise in the particular practice to create standards and review individual
cases. At the direction of the Committee, Board staff send quarterly threshold letters to each prescriber and dispenser who
has a patient that visited at least five prescribers and filled prescriptions in at least five pharmacies in a 90-day period and
recently began sending letters of concern to outlier/high-level prescribers requesting review of prescribing patterns and a
response to the Committee. Last year, at the suggestion of the legislature, the Committee began meeting monthly to more
proactively and regularly review K-TRACS data and identify patterns of concern. As part of this work, the Committee

2
approved specific guidelines on January 11, 2019 to aid staff in presenting K-TRACS data to the Committee to review
and make such referrals as may be necessary for the protection of the public. During 2019, the Committee reviewed a total
of 22 cases of concern. Based on discussion, 15 cases had letters sent to the appropriate entities, such as patient prescriber
or pharmacist, law enforcement, or other regulatory agency for intervention. Cases will continue to be reviewed by the
Committee in 2020 with the assistance of a new, grant-funded staff member to the Board of Pharmacy.
Operations, Funding, and Enhancements:
K-TRACS was implemented and operated using federal grant funds through June 30, 2016. The Board has now exhausted
available grant funding to sustain the program, and the only remaining grant funding is for program enhancements.
Program maintenance costs include the cost of software, staff, and office overhead (postage, paper, etc). While the Board
continues to pursue and has recently been awarded federal grants, funding presents the largest obstacle to maintaining a
PDMP in Kansas. In 2016, the Board received legislative approval to use approximately $200,000 of surplus dollars from
the pharmacy fee fund to cover operating expenses for FY2017. In 2017, the Board of Pharmacy, Board of Healing Arts,
Dental Board, Board of Nursing, and Board of Optometry sought and received legislative authority to use surplus fee fund
dollars to collectively support the program through FY2018 and FY2019. In 2019, the legislature authorized a transfer of
$705,000 from the KDHE Drug Manufacturer’s Rebate Fund in addition to continued and increased funding from the
aforementioned fee funds. A permanent funding solution continues to be a top priority of the K-TRACS program. The
table below represents the allocated amounts from FY2018 through FY2021.
Table 1. K-TRACS Transfers from Fee Funds, FY 2018 – FY2021
The Board employs an Assistant Director and a Program Manager to oversee and administer the PDMP. An Advanced
Epidemiologist is also funded through a recent federal grant through August 2022 to analyze K-TRACS data and provide
necessary reporting under the federal grants. In addition to daily administrative and operational duties, staff members
make regular presentations on the PDMP to prescribers, pharmacists, public health professionals, and other organizations.
Additional administrative support is provided by Board of Pharmacy licensing staff. Human resources and staff
availability limit significant expansion of the program, grant applications/awards, customer service, awareness campaigns,
and other program analysis and review. The Board is currently working to hire three additional, temporary, K-TRACS
staff members through September 2021 using Harold Rogers grant funds (see explanation below). These positions include
a Public Information Officer to create training and awareness materials for users and members of the public; a Program
Specialist to examine the accuracy and timeliness of data reported to K-TRACS for compliance with the Prescription
Monitoring Program Act; and a Physician or Pharmacist to review K-TRACS data for suspicious patterns or activity, and
referral to the Advisory Committee for consideration and possible action.
The Board began collecting data in February 2011 and the program became fully operational in September 2011. In July
2013, Kansas became the first state to launch a pilot of new software called AWARxE™ hosted by the National
Association of Boards of Pharmacy (NABP) through Appriss Health, Inc., which was offered at no charge through June
30, 2016. The Board now contracts directly with Appriss for the maintenance, support, and hosting of K-TRACS
software. Appriss is the vendor for 43 PDMPs and provides a strong PDMP solution. In FY 2018, Appriss identified a
need for greater transparency in their software planning and releases. Since that time, all PDMP administrators now have
access to an interactive Product Roadmap which outlines past and future product improvements, fixes, and enhancements
developed by other states and made available to all clients. The Board continues to have an excellent working relationship
with Appriss. Regular check-in calls are scheduled with the K-TRACS staff and in-person meetings occur annually with
Providers FY18 Actual FY19 Actual FY20 FY21
BOHA 11,788 83,945.64$ 81,847.91$ 235,500.00$ 235,500.00$
Nursing 5,301 30,704.52$ 36,806.56$ 103,500.00$ 103,500.00$
Dental 2,032 13,442.32$ 14,108.84$ 41,500.00$ 41,500.00$
Optom 695 4,694.15$ 4,825.61$ 16,500.00$ 16,500.00$
Pharm 6,527 39,120.38$ 45,319.08$ 130,500.00$ 130,500.00$
Drug Rebate Fund 705,000.00$ 705,000.00$
Totals 26,343 171,907.00$ 182,908.00$ 1,232,500.00$ 1,232,500.00$

3
the team. Inquires for technical assistance and customer support are generally responded to within one or two business
days.
The AWARxE™ platform accommodates large chains, independent and small pharmacies, and works seamlessly with the
National Association of Boards of Pharmacy (NABP) - PMP Interconnect® (PMPi) which is offered at no charge by
NABP. PMPi is a system which facilitates the transfer and availability of PDMP data to all participating states (48
available, Figure 1). As of December 31, 2019 Kansas is sharing data with 34 states, districts, and territories, and also now
shares data with the Military Health System, Indian Health System, and Veterans Administration. The neighboring state of
Oklahoma is Kansas’ largest requester of K-TRACS data, followed by Texas and Missouri.
Figure 1. Jurisdictions connected with the National Associations of Boards of Pharmacy PMP InterConnect (PMPi) as of
December 2019
Figure 2. Total number of K-TRACS successful PDMP searches by PMPi connected states.
The Board received a grant in 2012 from the Substance Abuse and Mental Health Services Administration (SAMSHA)
through the U.S. Department of Health and Human Services which funded integration of K-TRACS data into the Lewis
and Clark Information Exchange (LACIE) and Via Christi Health Systems, enabling a single sign-on for access to a
patient’s medical record and K-TRACS history. The Board, in conjunction with KDHE, has been expanding that project
to provide interoperability services for all prescribers and pharmacists in Kansas to access K-TRACS through the PDMP
Gateway®. This program, called INTEGRx8, is an opportunity for Kansas to deliver a more efficient and patient-oriented
program. The project was funded by a grant from the Centers for Disease Control (CDC) awarded to KDHE from 2017 –
2019 and will continue to receive partial support from a new CDC grant awarded to KDHE through August 2022. Grant
Graphic Source: https://nabp.pharmacy/wp-content/uploads/2019/04/PMP-InterConnect-Map-August-2019.pdf
Graphic Source: https://analytics.apprisshealth.com/#/site/USA_KS/views/PMPi/DisclosuresMap as of October 29, 2019
2018
2019

4
funds will support INTEGRx8 for each Kansas electronic health records and pharmacy management system approved for
integration which will further the K-TRACS mission. Statewide integration increases availability, ease of access, and use
of a patient’s-controlled substance prescription history for making critical and informed prescribing and dispensing
decisions. If prescriber’s and pharmacist’s electronic systems are not currently integrated, they are required to log in to
separate systems to query patient data which takes valuable time away from patient care and interaction. INTEGRx8
simplifies the process by creating a one-stop-shop, making K-TRACS data directly available in the patient’s electronic
record, and saving 4.22 minutes per patient on average. As of November 2019, a total of 794 entities were integrated with
K-TRACS through the INTEGRx8 initiative. Figure 3 below further breaks down the type of entity and total number that
are integrated and Figure 4 graphically displays the impact of INTEGRx8 project on the proportion of Gateway® patient
searches per opioid prescription, showing an increase from 0.7 searches per prescription in July 2017 to 7.0 patient
searches per opioid prescription in July 2019. This increase signals increased collaboration in clinical decision making
among both prescribers and pharmacists to improve health care coordination for the patient.
Figure 3. Summary of K-TRACS INTEGRx8 progress as of November 2019
Figure 4. Timeline of interoperability and integration projects through the PMP Gateway and impact of prescriber and
pharmacy patient searched (Jan 2016 – July 2019)
5
The newest CDC grant awarded to KDHE has resulted in a continued partnership with the Board from January 2020 –
August 2022. Funding for January 2020 – August 2020 totals $981,845.84. While subrecipient funding awarded to the
Board does not replace funds previously allocated by the legislature, this funding will help support continued integration
efforts (described above) and funding for the following grant objectives:
• 1.0 FTE – Advanced Epidemiologist
• Travel to Required Grant Meetings
• Advanced Analytics Software Package
• Deidentified Data Extractions from K-TRACS
• Peer to Peer Learning National Meeting
The Board is also a recent recipient of a U.S. Department of Justice, Bureau of Justice Assistance, Harold Rogers grant in
the amount of $736,313.00 for the project period December 2019 – September 2021. Again, the award will not replace
funds previously allocated by the legislature, but will support temporary enhancements to the K-TRACS program. The
Board’s grant objectives include adding 3.0 FTE (temporary) as described above, travel to required grant meetings,
mandatory connection to the federal PDMP data-sharing hub known as RxCheck, and supplies, software, and equipment
for the new positions.
Alongside efforts to increase K-TRACS utilizations through the INTEGRx8 program, individual user registration has also
continued to increase. Table 2 below summarizes the number of
prescribers and pharmacists registered in K-TRACS, which
has almost doubled since December 2013. Current users represent approximately 71% of the active controlled substance
prescriber community in Kansas and approximately 74% of the active pharmacist community in Kansas. Currently, the
use of K-TRACS is not mandatory in Kansas.
Table 2. Total number of
prescribers and pharmacists registered in K-TRACS (December 2013 – October 2019)
12/2013
12/2014
12/2015
12/2016
12/2017
12/2018
10/2019
Registered Prescribers
5,287
5,724
6,747
7,755
8,968
9,428
10,175
Registered
Pharmacists
1,042
1,272
1,652
1,976
2,400
2,886
3,195
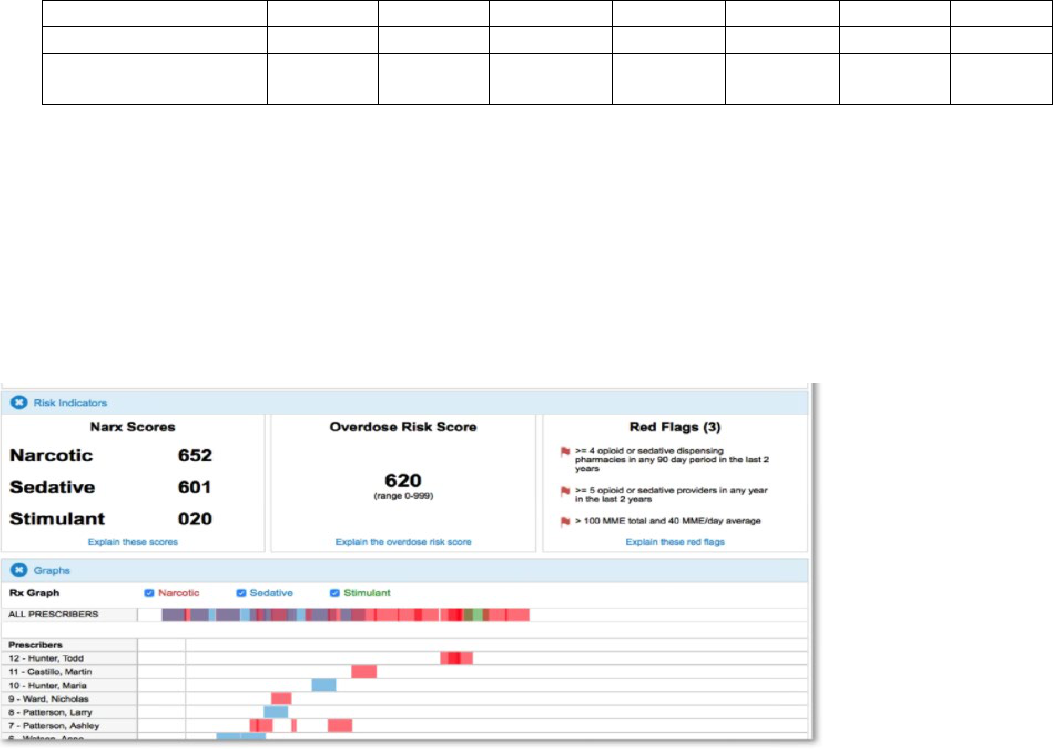
NarxCare is the newest upgrade to the K-TRACS system beginning January 2019. NarxCare provides patient and clinical
decision support beyond the patient’s prescription history by: 1) compiling multiple state reports into one cohesive profile;
2) analyzing data to provide reports, use scores, predictive scores, red flags, visualizations, and K-TRACS data including
narcotics, sedatives, and stimulants; 3) including resources such as Medication Assisted Treatment (MAT) locators and
Centers for Disease Control and Prevention printable educational handouts; and 4) creating Care Team Communications,
perhaps the most powerful tool within NarxCare in the prevention and treatment of substance use disorder coordination of
care (Figure 5).
Figure 5. NarxCare report summary

6
K-TRACS Impact in Kansas:
The opioid crisis continues to impact the United States; Kansas is no exception. However, progress is being made. For the
first time since the opioid epidemic began in the 1990’s, the Department of Health and Human Services (HHS) reported a
5.1% decline in drug overdoses during the 12-month period between 2017 and 2018 (Source: US Health and Human
Services (HHS Statement)
). However, during this same time frame, Kansas observed an increase in the number of drug-
related overdose deaths by 6.1% (figure 6). In the past 10 years, Kansas drug-related overdose deaths have increased by
15.7%. In addition, CDC has highlighted growing concern related to increases in stimulant use and poly substance use,
which may be contributing to a fourth wave of the US drug crisis (Source: Boston Medical Center, Health System (
BMC
source article)). This trend has also been observed in Kansas, with psychostimulant deaths increasing by 413.3% over the
past 10 years from 15 in 2009 to 77 in 2018. K-TRACS data also indicates prescribing of stimulants in Kansas has
increased 14.7% from 2015 to 2018. It is also noteworthy that many death certificates in Kansas list multi-drug toxicity as
a contributing cause of death which may lead to underreporting of death attributed to controlled substances overall. While
declining, in 2018, 58 deaths were classified as unspecified drug overdose and 181 reported unknown cause of death.
Figure 6. Age adjusted mortality rates attributed to drug poisoning per 100,000 Kansas residents (2005-2018)
Note: Rates based on small cell counts are generally thought to be unstable. The Kansas Department of Health and Environment define
small cell counts as less than 6 events. The only category impacted by this suppression rule is Heroin for the time frame 2005-2007, which is
not displayed in this figure.
In Kansas, controlled substance prescribing is changing. Overall, the number of Kansas patients receiving opioid
prescriptions with greater than or equal to 100 MME has declined by 35% from first quarter 2018 to third quarter 2019
(table 3). Despite this, the percent of opioid naive patients receiving long-acting opioid prescriptions has increased
steadily from first quarter 2018 (5.13%) to third quarter 2019 (6.10%) (table 3). Benzodiazepine prescribing has also
declined by almost 10% from 2015 (table 4). It is thought these changes may be attributed to both national and state level
provider education efforts. These efforts are continuing as a result of federal grant programs and statewide initiatives.
Table 3. Summary of Opioid Dispensation Reported to K-TRACS, January 2018 – October 2019
Q1
2018
Q2
2018
Q3
2018
Q4
2018
Q1
2019
Q2
2019
Q3
2019
Number of Kansas Patients Receiving more
than an average daily dose of >= 100 MME
17,331
16,063
14,608
13,834
12,904
11,962
11,264
Number of opioid prescriptions greater than
100 total MME
490,538
483,581
465,238
459,140
434,000
430,142
422,707

7
Percent of patient receiving more than an
average daily dose of morphine milligram
equivalent of 90 or greater (across all in state
opioid prescriptions)
11.64%
8.70%
8.20%
8.00%
7.80%
7.20%
6.80%
Percent of Long Acting or Extended Release
Opioid Prescriptions Dispensed to an Opioid
Naive Patient
5.13%
5.30%
5.30%
5.50%
5.80%
5.60%
6.10%
Percent of prescribed opioid days that
overlap with a benzodiazepine prescription
18.26%
19.10%
18.00%
17.80%
17.20%
16.40%
15.30%
Rate of Multiple Provider Episodes for
prescription Opioids (5 or more prescribers
and 5 or more pharmacies per 100,000
residents)
11.9
10.8
8.8
8.6
5.6
6.0
6.1
Table 4. Total number of prescriptions dispensed in Kansas, as reported to K-TRACS for select substances (2015-2018)
2015 2016 2017 2018
Percent
Change from
2015 to 2018
Total opioid Prescriptions
2,537,373
2,584,622
2,379,221
2,163,908
-14.7
Total Stimulant Prescriptions
667,774
731,538
755,611
766,236
14.7
Total Benzodiazepine
prescriptions
1,151,720
1,194,996
1,136,395
1,041,203
-9.6
In addition to controlled substances, K-TRACS monitors other drugs of concern in Kansas, identified by the Board in
Kansas Administrative Regulation 68-21-7. In FY 2018, the Board amended K.A.R. 68-21-7 to include the drug
“gabapentin” as a drug of concern. This change is the result of similar scheduling in surrounding states and significant
evidence of abuse and misuse by patients in recent years, often resulting in death. Gabapentin reporting in K-TRACS has
increased dramatically since the regulation change effective July 25, 2018 (Table 5). In 2018, four deaths occurred in
which gabapentin was listed as a contributing cause of death.
Table 5. Summary of Gabapentin reporting to K-TRACS by fiscal year (2015-2019)
FY 2015
FY 2016
FY 2017
FY 2018*
FY 2019*
Total Number of Gabapentin Prescriptions
Reported to K-TRACS
10 1,134 2,616 75,714 821,786
Total Number of Patients with a Gabapentin
Prescription
7 296 618 58,384 247,045
*Gabapentin became a drug of concern in Kansas as of 2018. Mandatory reporting of gabapentin dispensations to K-TRACS was implemented July
25, 2018. Prior to this date, all reports of gabapentin dispensations to K-TRACS was voluntary.
Drug seeking behaviors among patients also seem to be declining. The rate of multiple provider episodes for prescription
opioids (5 or more prescribers and 5 or more pharmacies per 100,000 residents) has dramatically declined from first
quarter 2018 (11.9 per 100,000) to third quarter 2019 (6.1 per 100,000) (Table 3). However, deaths associated with drug
overdoses among Kansas residents has increased by 44% since 2005. Also, a special analysis of 158 Kansas deaths
associated with prescription drug use, occurring from January 1, 2018 to May 1, 2019, found that 45.5% of decedents had
both benzodiazepine and opioids listed on the death certificate. These findings indicate that Kansas is still being
negatively impacted by the misuse of prescription drugs and illicit drug use, despite national declines.
Naloxone Dispensing in Kansas:
In 2017, after the passage of HB 2217, the Board adopted K.A.R. 68-7-23 which permits any licensed pharmacist to
dispense an emergency opioid antagonist and necessary medical supplies needed to administer an emergency opioid
antagonist to a patient, bystander, first responder agency or school nurse without a prescription, in accordance with a
physician-approved statewide protocol. The protocol summarizes indications for use of Naloxone, signs and symptoms of

8
an opioid-related overdose, including environmental signs, precautions, and contraindications for use of Naloxone,
documentation and record keeping procedures, and authorization for dispensations of Naloxone. To increase public
awareness of this protocol and where pharmacists are located throughout that state that have signed a Naloxone protocol,
an interactive Tableau map has been created for upcoming public dissemination on the Board website. Figure 7 is a screen
shot version of this map.
Figure 7. Screen shot of Interactive Naloxone Distribution Map

9
Ongoing Initiatives and Public Information:
Currently, the Board maintains a website for K-TRACS at
https://ktracs.ks.gov, with updated forms, frequently asked
questions/answers, and other helpful resources for healthcare workers and the public. K-TRACS data is available on the
PreventOverdoseKS.org website, where a Tableau Dashboard is embedded into the website. Plans are in place to update
the K-TRACS dashboard with current data in early 2020. In addition, with support from the aforementioned Harold
Rogers grant, the Board plans to implement a public media campaign focusing on best practices for K-TRACS use for
pharmacists and prescribers and increased dissemination of K-TRACS data for public consumption.
Respectfully submitted,
Alexandra Blasi, JD, MBA
Executive Secretary
