
Original Paper
Trial Promoter: A Web-Based Tool for Boosting the Promotion of
Clinical Research Through Social Media
Katja Reuter
1*
, PhD; Francis Ukpolo
1
, MBA; Edward Ward
1
, BS; Melissa L Wilson
2
, PhD, MPH; Praveen Angyan
1*
,
MS
1
Southern California Clinical and Translational Science Institute, Keck School of Medicine of USC, University of Southern California, Los Angeles,
CA, United States
2
Departments of Preventive Medicine & Obstetrics and Gynecology, Keck School of Medicine of USC, University of Southern California, Los Angeles,
CA, United States
*
these authors contributed equally
Corresponding Author:
Katja Reuter, PhD
Southern California Clinical and Translational Science Institute
Keck School of Medicine of USC
University of Southern California
CSC 200 Bldg, 2nd floor
2250 Alcazar Street
Los Angeles, CA, 90033
United States
Phone: 1 3234422046
Fax: 1 3234422082
Email: [email protected]
Abstract
Background: Scarce information about clinical research, in particular clinical trials, is among the top reasons why potential
participants do not take part in clinical studies. Without volunteers, on the other hand, clinical research and the development of
novel approaches to preventing, diagnosing, and treating disease are impossible. Promising digital options such as social media
have the potential to work alongside traditional methods to boost the promotion of clinical research. However, investigators and
research institutions are challenged to leverage these innovations while saving time and resources.
Objective: To develop and test the efficiency of a Web-based tool that automates the generation and distribution of user-friendly
social media messages about clinical trials.
Methods: Trial Promoter is developed in Ruby on Rails, HTML, cascading style sheet (CSS), and JavaScript. In order to test
the tool and the correctness of the generated messages, clinical trials (n=46) were randomized into social media messages and
distributed via the microblogging social media platform Twitter and the social network Facebook. The percent correct was
calculated to determine the probability with which Trial Promoter generates accurate messages.
Results: During a 10-week testing phase, Trial Promoter automatically generated and published 525 user-friendly social media
messages on Twitter and Facebook. On average, Trial Promoter correctly used the message templates and substituted the message
parameters (text, URLs, and disease hashtags) 97.7% of the time (1563/1600).
Conclusions: Trial Promoter may serve as a promising tool to render clinical trial promotion more efficient while requiring
limited resources. It supports the distribution of any research or other types of content. The Trial Promoter code and installation
instructions are freely available online.
(J Med Internet Res 2016;18(6):e144) doi: 10.2196/jmir.4726
KEYWORDS
algorithm; automation; clinical trial; communication; Facebook; Internet; online; patient; recruitment; social network; social
media; Twitter
J Med Internet Res 2016 | vol. 18 | iss. 6 | e144 | p. 1http://www.jmir.org/2016/6/e144/
(page number not for citation purposes)
Reuter et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX

Introduction
Scarce information about clinical research, in particular clinical
trials, is among the top reasons why potential participants do
not take part in clinical trials. Clinical trials are vital for the
development of novel approaches to advancing medicine, but
without volunteers this type of research is impossible. In 2012,
the Institute of Medicine recognized the seriousness of the
clinical trial participation problem [1] and released a report that
identified numerous barriers, including the lack of awareness
among patients and physicians that clinical trials are available.
New solutions are needed that increase clinical trial awareness
and build rapport among patients, physicians, and caregivers
with the aim to boost clinical trial engagement and recruitment
rates.
We have developed and tested Trial Promoter, a Web-based
tool that automatically generates and distributes social media
messages about clinical trials. New digital options such as social
media (ie, social networks) have the potential to work alongside
traditional methods to boost the promotion of clinical research.
Social media describe websites and Web-based applications
that enable users to create and share content or to participate in
social networking. With millions of users, social media serve
as a promising solution to improve the public awareness of
clinical trials and to support research participant recruitment
efforts. In fact, the use of the Internet as a top source for clinical
research information has increased significantly (46% in 2013),
whereas the use of mass media has declined (newspaper, radio,
television; 39% in 2013) [2]. Between 30% and 40% of the
public reports that they have used social media to gather medical
information to learn about clinical research, with the social
network Facebook topping the list [3-6]. This trend is not limited
to young adults; half of people aged 50 years and older and
more than a third of people aged 65 years and older frequent
social networking sites such as Twitter and Facebook [7-9].
These data suggest that patients, caregivers, and disease
advocates can be found, informed, and engaged digitally. We
have built Trial Promoter to leverage this digital trend and to
support research institutions that seek to respond to the evolving
way in which patients, physicians, caregivers, and advocacy
groups search for, create, and use health information online.
Trial Promoter builds on preliminary work where we tested an
automated approach to generate and publish messages about
research-related content on Twitter. Our work indicated that a
machine algorithm helps research teams and institutions to
increase the output and reach of information about research on
social media while reducing the burden of developing and
distributing hundreds of messages [10].
Here we present the tool and preliminary data, suggesting that
Trial Promoter may aid in distributing clinical trial information
more broadly while requiring limited resources. The tool serves
four functions: first, it imports information from specific
databases or data files. Second, it generates user-friendly social
media messages based on preapproved message templates.
Third, it schedules and distributes these messages through the
social media platforms Twitter and Facebook. Fourth, it tracks
the success of the messages and displays their engagement and
conversion metrics data. The source code and installation
instructions are freely available online [11]. In order to test the
tool, we conducted a 10-week trial. Trial Promoter randomized
46 active and recruiting clinical trials into social media messages
and distributed them via Twitter and Facebook. We assessed
the correctness of the test messages and calculated the
probability with which Trial Promoter generated accurate
messages.
Methods
Trial Promoter Setup
System Requirements
Trial Promoter is built using Ruby on Rails (version 4.2.1),
HTML, cascading style sheet (CSS), and JavaScript. We have
installed Trial Promoter on Ubuntu Linux 14.04 LTS (long-term
support) and use Phusion Passenger, a scalable Web server that
hosts Trial Promoter. Trial Promoter further uses PostgreSQL
9.4.5 database systems deployed on Ubuntu 14.04 LTS.
Administrator privileges for setting up Cron jobs are required
in order to set up nightly data extractions that secure logs, collate
metrics, and distribute messages.
Information Import
Trial Promoter has the capability to import information from
different databases and data files through either a
representational state transfer (REST) application programming
interface (API) or a comma-separated values (CSV) file. Figure
1 depicts the Trial Promoter setup and data flow. Figure 2
represents a screenshot of the local Trial Promoter interface that
shows imported clinical trial information and disease keywords
that were included in the test messages. Table 1 lists the
information our local Trial Promoter installation imported for
testing purposes, for example, clinical trial information from
our institutional Clinical Studies Directory that utilizes data
from ClinicalTrials.gov provide by the National Library of
Medicine [12], message templates designed for Twitter and
Facebook, and information on disease hashtags. Disease
hashtags are disease keywords preceded by a pound sign (eg,
#Diabetes, #BreastCancer). They are used by members of
disease communities on social media sites such as Twitter to
identify and discover messages on a specific topic [13,14].
J Med Internet Res 2016 | vol. 18 | iss. 6 | e144 | p. 2http://www.jmir.org/2016/6/e144/
(page number not for citation purposes)
Reuter et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX

Table 1. Data sources and types of data imported for testing purposes by our local installation of Trial Promoter.
Data typesData source/formatImported content
Clinical trial title
Clinical Studies Directory/REST API
a
Active, recruiting clinical trials
Name of principal investigator
Clinical trial landing page URL
Disease keywords
Symplur [11]/CSV
b
file
Disease hashtags
Text message templates designed for Twitter and
Facebook
N/A
c
/CSV file
Message templates
a
REST API: representational state transfer application programming interface.
b
CSV: comma-separated values.
c
N/A: not applicable.
Figure 1. Trial Promoter (TP) setup and data flow. The elements in blue represent functional TP modules. CSV: comma-separated values; REST API:
representational state transfer application programming interface.
J Med Internet Res 2016 | vol. 18 | iss. 6 | e144 | p. 3http://www.jmir.org/2016/6/e144/
(page number not for citation purposes)
Reuter et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX

Figure 2. Local Trial Promoter interface that shows imported clinical trial information and disease keywords that were included in the test messages.
Message Generation and Distribution
Trial Promoter Messenger develops social media messages
tailored to Twitter and Facebook. To achieve that, Trial
Promoter uses preapproved message templates that we
developed with input from communication experts at Keck
Medicine of the University of Southern California (USC).
During testing, we used 154 parameterized message templates.
Figure 3 represents a screenshot of the local Trial Promoter
interface that shows parameterized message templates for
Twitter and Facebook, which were used during testing. The
parameterization supports the generation of large sets of
messages from a limited set of clinical trials and message
templates. The words in italics the following example messages
represent parameters that Trial Promoter added into the message
templates to create the final social media messages.
Example of Twitter message template: “New #ClinicalTrial
@KeckMedUSC on #disease is looking for participants. Please
help us spread the word. Thx. URL ”
Example of Facebook message template: “Your help is
appreciated: New clinical trial at Keck Medicine of USC on
#disease is looking for participants. Through these types of
clinical studies researchers can better understand how to
diagnose, treat and prevent diseases. Please share this URL.
Thanks!”
Trial Promoter matches a randomly chosen clinical trial with a
randomly chosen message template using the standard Ruby
library to generate random numbers [15]. The random numbers
in the library are implemented as a modified Mersenne Twister
with a period of 2
19937
−1 [16]. Trial Promoter shuffles all clinical
trials and then randomly chooses a message template for each
trial [17], ensuring that all clinical trials are only distributed
once during a given time period.
Trial Promoter then substitutes the parameters in the message
templates and includes several weblinks into the message
templates to create the final messages: first, a tagged and
shortened URL that links the social media message to the
respective clinical trial landing page; second, a primary and if
applicable a secondary disease hashtag (eg, #LungCancer,
#SleepApnea); and third, for Twitter messages the official Keck
Medicine of USC Twitter account (@KeckMedUSC). Table 2
lists the characteristics of the messages that Trial Promoter
generated automatically during the testing phase.
J Med Internet Res 2016 | vol. 18 | iss. 6 | e144 | p. 4http://www.jmir.org/2016/6/e144/
(page number not for citation purposes)
Reuter et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX

Table 2. Characteristics of test messages that Trial Promoter generated automatically for distribution on Twitter and Facebook.
FacebookTwitterCharacteristic
N/A
b
Limitation to 140 characters
a
Maximum message length limitation
Links can be of any length. However, in order to simplify
URL sharing and present clean URLs to the Facebook page
visitor, Trial Promoter uses Bit.ly shortened URLs on Face-
book posts as well.
22 characters for non-https URLs
23 characters for https URLs
Parameter: URL
Yes (primary and secondary hashtags)Yes (primary and if length permits secondary
hashtag)
Parameter: hashtags (disease keyword)
N/A
b
YesParameter: link to official Keck
Medicine of USC
c
Twitter account
(@KeckMedUSC)
a
Note: Media attachments such as photos, videos, and polls are not counted toward 140 characters.
b
N/A: not applicable.
c
USC: University of Southern California.
Furthermore, Trial Promoter tags the URL that links to the
clinical trial landing page with Urchin Traffic Monitor (UTM)
parameters in order to track the link engagement (or clicks) on
social media and referral traffic to the clinical trial landing page
[18]. During testing, Trial Promoter used the REST API
provided by the Bit.ly link-shortening service to generate the
shortened URL [19]. Bit.ly preserves the UTM parameters by
mapping identical links with different UTM parameters to
unique URLs.
For Twitter, the automated inclusion of disease hashtags that
vary in length depending on the disease term used may result
in messages that are longer than 140 characters (eg, #HIV vs
#PancreaticCancer). If the generated message was greater than
140 characters, Trial Promoter discarded the message and
selected an alternative message template until it either generated
a valid message or it ran out of message templates to choose
from. In the latter case, Trial Promoter notified the study team
of the error in the administrative dashboard.
Trial Promoter Messenger then schedules and distributes the
test messages through selected Twitter and Facebook accounts
(eg, USC Clinical Trials) using the social media content
management Web application Buffer [20]. Each social media
account set up in Buffer has a unique profile identifier (ID)
assigned to it. Buffer provides a REST API call that allows for
programmatic scheduling of messages directly in Buffer. With
a single call, the Buffer REST API [21] sends a single message
to multiple social media channels. The REST API provides
options for scheduling messages and including images. Once
messages are sent to Buffer, the tool interface allows users to
edit, reschedule, reorder, and delete messages.
Figure 3. Local Trial Promoter interface shows parameterized message templates for Twitter and Facebook that were used during testing.
J Med Internet Res 2016 | vol. 18 | iss. 6 | e144 | p. 5http://www.jmir.org/2016/6/e144/
(page number not for citation purposes)
Reuter et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX

Automated Tracking of Message Success
Trial Promoter Collector tracks key performance indicators
(KPIs) associated with the success of the messages, that is,
social media engagement and landing page conversion data.
Table 3 lists the engagement and conversion KPIs tracked by
Trial Promoter. Trial Promoter Collector extracts these data on
a nightly basis from 4 existing systems: Buffer, Twitter,
Facebook, and Google Analytics [22]. The tool connects to
Buffer via a REST API in order to extract the social media and
link engagement data for each social media message sent via
Buffer. To collect the data from Twitter and Facebook, our team
uploaded reports taken from each social media platform to Trial
Promoter. Trial Promoter processed these raw logs and imported
them into its dashboard on a nightly basis. The landing page
conversion data are accessible through Google Analytics. To
extract the data, Trial Promoter required uploads of the Google
Analytics data report into Trial Promoter. Trial Promoter then
processed and incorporated the data into its dashboard.
Table 3. Engagement and conversion key performance indicators tracked by Trial Promoter.
Measures on FacebookMeasures on TwitterMetric categories
ImpressionsImpressionsVolume of messages served
Shares
Comments
Likes
Retweets
Replies
Likes
Social media engagement
Clicks from social media message to clinical
trial landing page on Clinical Studies Directory
Clicks from social media message to clinical trial
landing page on Clinical Studies Directory
Link engagement
Sessions
Time spent on page
Pageviews per visit
Sessions
Time spent on page
Pageviews per visit
Landing page engagement
Contact form usage on individual clinical trial
information page
Contact form usage on individual clinical trial in-
formation page
Contact engagement
Finally, Trial Promoter Dashboard displays the KPI data,
providing daily updates. Figure 4 represents a screenshot of the
local Trial Promoter interface that shows KPI data for each
Twitter message. During testing, Trial Promoter matched up
data from the raw data logs for each social media platform to
the data from Buffer using a unique social message (or update)
ID. Each social media channel generates a unique ID for every
message that is sent out via its platform. Using this unique ID
allowed us to match up entries in the raw data logs to a specific
message. Additionally, the Trial Promoter Dashboard serves as
a control panel to add, edit, and delete clinical trials, message
templates, and social media messages and images.
Trial Promoter Evaluation
Test Trial Design
During the 10-week test trial, Trial Promoter randomized clinical
trials (n=46) into social media messages using preapproved
message templates. The tool generated, scheduled, and published
2 messages on each platform (Twitter and Facebook) per day,
and 3 messages per platform every other day.
Correctness Analysis
The correctness with which Trial Promoter generated messages
during the 10-week testing phase was evaluated using 4
indicators: (1) the correct usage of the message template, (2)
the text of the message itself (ie, number of text errors in the
message), (3) the inclusion of the correct URL, and (4) the
inclusion of the correct disease hashtags. The individual
indicators were averaged to obtain the overall percent correct.
The correct usage of the message template was measured
through random sampling 25/525 messages (5%) and manually
comparing the message template with the social media that was
generated by Trial Promoter. The correctness of the included
URL was evaluated using a script written in Ruby on Rails [8].
The script examined the URL by first expanding the shortened
Bit.ly URL to a complete URL. The URL was then compared
with the landing page of the clinical trial being promoted using
regex expressions (ignoring any query strings in the URL) to
ensure that they were identical. The inclusion of the correct
disease hashtags (eg, #Stroke, #LungCancer) was manually
reviewed in all 525 messages upon scheduling.
J Med Internet Res 2016 | vol. 18 | iss. 6 | e144 | p. 6http://www.jmir.org/2016/6/e144/
(page number not for citation purposes)
Reuter et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX

Figure 4. Local Trial Promoter interface shows key performance indicator data for each message. This example shows Twitter messages.
Results
Trial Promoter Evaluation
During the 10-week testing phase, Trial Promoter successfully
generated and distributed 175 messages via Twitter and 350
messages via Facebook, a total of 525 messages. Figure 5 shows
examples of messages that Trial Promoter generated
automatically for distribution on social media.
Correctness Analysis
The analysis of the test messages revealed that in a random
sample of 25/525 messages the Trial Promoter algorithm used
the correct message template without any text errors 100% of
the time. However, we discovered a recurring issue in 13/525
messages (2.5%) where the algorithm used a question mark
instead of an apostrophe (eg, “We’re looking for participants”).
Furthermore, the analysis of the URLs showed that 525/525
messages (100%) included the correct URL. Finally, the disease
hashtag analysis revealed that 24/525 distributed messages
(4.6%) lacked the disease hashtag. Trial Promoter had not
substituted the parameter “#disease” in the message template
with the disease of the clinical trial in all cases. On average,
Trial Promoter correctly used the message templates and
substituted the message parameters (text, URLs, and disease
hashtags) 97.7% of the time (1563/1600).
Availability
The Trial Promoter software code is available under the MIT
license [22,23]. Software code versions for technical and
nontechnical users are accessible through the Trial Promoter
website and hosted on GitHub [10]. To simplify the installation
process for nontechnical users, we have written a script that
deploys an instance of Trial Promoter to the public hosting
service Heroku—within less than 30 minutes and without
requiring technical knowledge of server setup and system
administration. A fee of US $14 per month is required for
hosting Trial Promoter on Heroku. Using the provided code,
nontechnical users will be able to do the following: add clinical
trial information for promotion—one trial at a time; import
message templates—one at a time; automatically integrate with
the social media management tool Buffer that automates the
distribution of the messages; and create a dashboard that imports
metrics from Buffer via a REST API, and from Twitter and
Facebook via a CSV file. For technical users, detailed
instructions for hosting Trial Promoter on an Ubuntu 14.04 LTS
machine are also available on GitHub. Using the provided code,
technical users will be able to do the following: import clinical
J Med Internet Res 2016 | vol. 18 | iss. 6 | e144 | p. 7http://www.jmir.org/2016/6/e144/
(page number not for citation purposes)
Reuter et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX

trial information via a REST API—many trials at once; import
message templates via a REST API—multiple templates at
once; automatically integrate with Buffer that automates the
distribution of the messages; and create a dashboard that
automatically imports metrics from Buffer via a REST API,
and that imports metrics from Twitter and Facebook via a CSV
file. Finally, the full code used in the experiment described here
is accessible through the Trial Promoter website and viewable
on GitHub [10].
Figure 5. Examples of messages that Trial Promoter generated and published automatically.
Discussion
Automated Clinical Trial Promotion
We have developed and tested Trial Promoter, a Web-based
tool that automatically generates and distributes user-friendly
social media messages about clinical trials. We chose the social
media platforms Facebook and Twitter because members of
disease communities frequently use them [2,24,25].
Trial Promoter managed to generate and distribute social media
messages with a high level of correctness. We were able to
improve the Trial Promoter algorithm with regard to the
technical issues we encountered during testing. First, in 5% of
the messages the algorithm had not substituted the generic
disease hashtag parameter (#disease) with the corresponding
disease hashtag taken from the clinical trial. We found that the
substitution algorithm was both case and white-space sensitive
(ie, # Disease, # disease, and #Disease). As a result, only the
parameter #disease without a space and with lowercase “d” was
replaced with the actual disease term that was associated with
a clinical trial. We have modified the Trial Promoter algorithm,
rendering the replacement of disease hashtags case and
white-space insensitive, thereby resolving the issue for the
future.
In light of millions of messages divulged by social media users
on Twitter and Facebook every day, tailoring those messages
to a specific audience is critical to cut through the noise.
Hashtags provide a useful tool to target messages to specific
topics and disease communities. The simple # symbol, known
as a hashtag, for example, #leukemia, #rheum, is included in
each message to indicate a topic, conversation, or event on
Twitter [14] that the message relates to. Our ongoing work is
focused on assessing the effectiveness of Trial Promoter for the
promotion of clinical trial messages through a new Twitter
account without followers. Following a Twitter user means to
subscribe to a person’s feed, that is, stream of messages.
Second, in 2.5% of the messages the algorithm introduced a
question mark instead of an apostrophe owing to encoding
issues. This error was introduced while copying the message
templates from a Google Docs file [24] into the code of our
local Trial Promoter installation that we used during the testing
J Med Internet Res 2016 | vol. 18 | iss. 6 | e144 | p. 8http://www.jmir.org/2016/6/e144/
(page number not for citation purposes)
Reuter et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX
phase. We consider this a rare technical issue specific to Google
Docs where an apostrophe is encoded differently than text within
a code editor. However, if necessary this type of technical issue
can be addressed and corrected through the administrative
dashboard of Trial Promoter.
The data thus far indicate that Trial Promoter serves as a
promising tool to support clinical trial promotion via social
media. More specifically, Trial Promoter is designed to facilitate
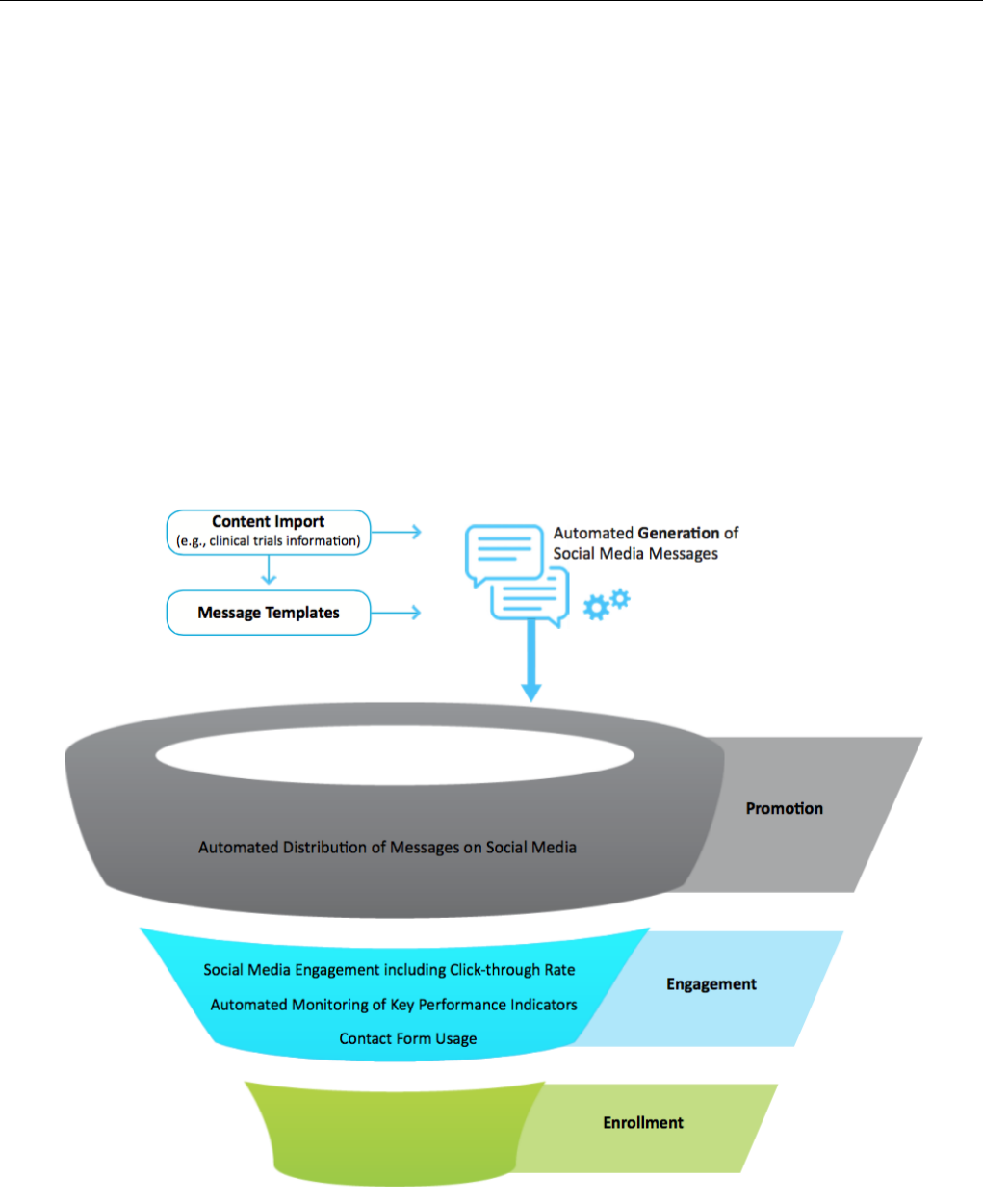
two phases of the clinical trial recruitment process. Figure 6
illustrates these phases: the promotion (or advertisement) phase
and the engagement phase. By publishing messages about
clinical trials on social media, Trial Promoter supports the
awareness-building phase of the clinical trial recruitment
process. Several studies indicate that awareness changes
attitudes toward clinical trials, enrollment, and the benefits of
participation. More than 80% of patients were either unaware
or unsure that participation in a clinical trial was an option at
the time of diagnosis, and 75% of these patients said they would
have been willing to enroll had they known it was possible
[25,26]. These data indicate that improving the distribution of
clinical trial information at limited cost may benefit clinical
trial recruitment efforts.
Trial Promoter further generates opportunity for social media
engagement by potential study participants, disease advocates,
and others because social media is designed to facilitate
interaction and conversation, for example, sharing, liking,
following, and replying. The link engagement in the message
that Trial Promoter generates and distributes is essential to triage
visitors to the clinical trial landing page where they can find
more information about the trial and potentially contact the
study team using a compliant contact form.
Figure 6. Trial Promoter is designed to facilitate two phases of the clinical trial recruitment process: the promotion (advertisement) and engagement
phases.
Saving Time and Cost
We wondered whether Trial Promoter could increase fiscal
efficiencies of the clinical trial promotion. This is especially
relevant in light of the high operational costs associated with
clinical trials [4,27]. Nearly 30% of the time dedicated to clinical
trials is spent on promotion, patient recruitment, and enrollment
[28]. Despite this substantial amount of time and cost, more
than 30% of all clinical trials fail to meet their enrollment
targets, and more than 10% never enroll a single patient [29].
To test the theory as to whether Trial Promoter makes the
promotion of clinical trials on social media more efficient
fiscally, we compared the labor and cost of Trial Promoter with
the labor and cost of a social media manager. During the
10-week testing phase, Trial Promoter automatically generated
525 user-friendly messages. The generation of a social media
J Med Internet Res 2016 | vol. 18 | iss. 6 | e144 | p. 9http://www.jmir.org/2016/6/e144/
(page number not for citation purposes)
Reuter et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX

message takes an average of 5-15 minutes including selecting
the content, writing the message, adding an optional image or
video, and posting the message (based on internal analysis).
According to jobs and recruiting site Glassdoor, the national
average annual salary for a social media manager in 2015 was
US $52,000 [30]. A social media manager would have required
43-131 hours of labor equivalent of US $1800-$5400 labor cost
to generate the number of messages Trial Promoter has
generated in 10 weeks (525). We argue that Trial Promoter helps
to decrease the time and cost of labor required to generate and
distribute information about clinical trials through social media.
Although there were costs associated with the development of
Trial Promoter (ie, personnel cost, server cost), those are
minimal in comparison to the fact that the tool will be a free
resource to the research community. It can be used repeatedly
at no additional cost to investigators and research institutions.
The tool may also help research institutions and investigators
to gain efficiencies by streamlining and improving the clinical
trials infrastructure and process so that those investigating new
research questions could quickly draw on resources already in
place instead of reinventing the wheel for each trial—in this
case the broader dissemination of clinical trial information via
social media.
Saving time and cost would not—in part—be possible without
automation. However, the use of automated postings in digital
marketing and social media is a controversial topic. We believe
automation to serve patients, disease advocates, and research
institutions alike when used appropriately and as long as the
content is relevant and of value to the audience. Trial Promoter,
for example, allows clinical study teams and others in charge
of promoting clinical trials to design and write the posts at times
when it is more convenient for them. Trial Promoter thereby
not only saves them time, the tool also schedules and
disseminates the social media posts at times when it is more
convenient for the audience.
Related Work
A number of studies have discussed the automation of the
production of news and information. Automation offers new
possibilities for creating content at scale, more quickly than a
human could. So-called “bots,” that is, automated accounts on
digital and social media (eg, Twitter, Facebook, Reddit, and
Wikipedia) that distribute news and information, have been
observed and studied in a variety of contexts: in social networks
and human communication decisions [31,32], social shaping
[33], content pollution [34], social metric gaming [35], ranking
manipulation [36], infiltration [37], political astroturfing [38],
recommendation [39], scholarship dissemination [40], activism
or advocacy [41], and journalism [42]. Lokot and Diakopoulos
concluded that news bots might enable innovation, such as niche
and local news [42]. Different definitions have been introduced
to describe these bots as “automated social actors”—software
designed to act similarly to how humans might act in social
spaces [31], as “software agents that interact on social
networking services” [33], and as “automatic or semi-automatic
computer programs that mimic humans and/or human behavior”
[43]. However, future research will need to investigate how the
public perceives news and information bots, whether they
recognize bots as automated information services, if they are
skeptical of content shared by a bot, and whether bots are
ultimately effective in achieving the bottom line, for example,
increase clinical study recruitment or foster the accessibility of
public health information. Some small-scale work suggests that
“while the software-generated content is perceived as descriptive
and boring, it is also considered to be objective although not
necessarily discernible from content written by journalists” [44].
Another study found that a Twitter bot sharing public health
information was perceived as “credible,” “attractive,” and
“competent,” suggesting that such “bots could be gainfully
employed by [organizations] if properly harnessed” [45]. The
authors are not aware of similar research that has tested the
feasibility and effectiveness of automated postings of clinical
research information. Yet the preliminary data in other fields
look promising.
Limitations
Limitations of the Study
The test trial we present here was focused on assessing the
probability with which Trial Promoter generates and distributes
correct messages about clinical trials. Future studies will be
required to systematically assess the efficacy of Trial Promoter
(or other algorithms) beyond fiscal efficiencies, determining
the ability of machine-generated clinical trial information to
foster the awareness of and engagement among target audiences
such as patients, disease advocates, and physicians. Furthermore,
it will be interesting to explore to what extent social media
engagement with machine-generated content translates into
increased clinical trial recruitment and enrollment rates.
Limitations of Trial Promoter
The current version of Trial Promoter does not automatically
include images into the social media messages. Images,
however, have been shown to be an important aspect of social
media messages to increase engagement and information uptake
[46]. The tool also does not automatically include mentions of
influencers in the messages, that is, names of Twitter users with
a lot of followers and reach—an important technique to increase
the exposure of messages among target audiences. Additionally,
Trial Promoter does not yet take into account awareness months
when scheduling messages, for example, October is Breast
Cancer Awareness Month. To increase the reach of the
distributed information, Trial Promoter could increase the
promotion of disease-related messages during awareness months.
Conclusions
In summary, we present Trial Promoter and preliminary data
indicating that the tool reliably automates the generation and
distribution of correct clinical trial messages via social media.
The Trial Promoter software code is freely available online.
Although our local installation and pilot project focuses on
clinical trials, Trial Promoter has the capability to support the
generation and distribution of any type of content. Other
examples of content include research news stories,
peer-reviewed articles, and information about research experts
and their expertise.
We hypothesize that machine-generated content helps research
institutions and investigators to distribute clinical trial
J Med Internet Res 2016 | vol. 18 | iss. 6 | e144 | p. 10http://www.jmir.org/2016/6/e144/
(page number not for citation purposes)
Reuter et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX

information more broadly and effectively. However, further
studies around machine-generated content on social media will
help to understand its role in facilitating patient engagement,
increasing clinical trial awareness, and improving study
recruitment and retention rates.
Acknowledgments
This work has been supported by the Southern California Clinical and Translational Science Institute and its Biostatistics program
through grant UL1TR000130 from the National Center for Advancing Translational Sciences of the National Institutes of Health
(formerly by the National Center for Research Resources, Award Number UL1RR031986). The content is solely the responsibility
of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors would like to thank the following individuals for their contributions, advice, and helpful feedback on the project and
manuscript as well as testing of Trial Promoter: Thomas A Buchanan, Bruce Park, Julian Lee, Sabrina Eng, and Melanie Funes.
Conflicts of Interest
None declared.
References
1. Public Engagement and Clinical Trials: New Models and Disruptive Technologies: Workshop Summary. Washington, DC:
National Academies Press (US); 2012.
2. The Center for Information & Study on Clinical Research Participation. 2013. Report on clinical trial information seekers.
Perceptions and insights study URL: https://www.ciscrp.org/ [accessed 2016-05-26] [WebCite Cache ID 6hn2TkTmy]
3. The Center for Information & Study on Clinical Research Participation. 2015. Report on the decision to participate.
Perceptions and insights study URL: https://www.ciscrp.org/ [accessed 2016-05-26] [WebCite Cache ID 6hn2acue8]
4. Taylor H, Leitman R. Misconceptions and lack of awareness greatly reduce recruitment for cancer clinical trials. Harris
Interactive: Health Care News 2001;1(3):1-3.
5. Fox S, Jones S. Pew Research Center. The Social Life of Health Information URL: http://www.pewinternet.org/files/
old-media/Files/Reports/2009/PIP_Health_2009.pdf [accessed 2016-02-18] [WebCite Cache ID 6fNNA4ZdY]
6. Facebook. URL: https://facebook.com/ [accessed 2016-02-17] [WebCite Cache ID 6fNFRSsU0]
7. Smith A. Pew Research Center. Older adults and technology use URL: http://www.pewinternet.org/2014/04/03/
older-adults-and-technology-use/ [accessed 2016-02-17] [WebCite Cache ID 6fNFn66n4]
8. Fox S, Duggan M. Pew Research Center.: Pew Research Center; 2013. Health Online 2013 URL: http://www.pewinternet.org/
2013/01/15/health-online-2013/ [accessed 2016-02-17] [WebCite Cache ID 6fNNRtbVO]
9. Twitter. URL: https://twitter.com/ [accessed 2016-02-17] [WebCite Cache ID 6fNFYrrhm]
10. Reuter K, Chatterjee A, Daigre J, Voytek B. Spreading research and engaging disease communities: One automated tweet
at a time. 2014 Presented at: Proceedings of the Joint Summits on Translational Science; April; 2014; San Francisco, United
States p. 7-11.
11. Trial Promoter. 2016. URL: http://trialpromoter.org [accessed 2016-02-17] [WebCite Cache ID 6fMwI58NC]
12. Clinicaltrials.gov. U.S. National Institutes of Health URL: http://clinicaltrials.gov/ [accessed 2016-02-17] [WebCite Cache
ID 6fNHGi4Zu]
13. Symplur. Healthcare Hashtag Project URL: http://www.symplur.com/healthcare-hashtags/ [accessed 2016-02-17] [WebCite
Cache ID 6fNG11EjX]
14. Harmel M, Young K. e-Patients in twitter hashtag communities. J Participat Med 2013;5:e22.
15. Ruby documentation project. URL: http://ruby-doc.org/core-2.2.0/Random.html [accessed 2016-02-16] [WebCite Cache
ID 6fNHNVE0H]
16. Matsumoto M, Nishimura T. Mersenne twister: a 623-dimensionally equidistributed uniform pseudo-random number
generator. ACM Trans Model Comput Simul 1998;8(1):3-30. [doi: 10.1145/272991.272995]
17. Ruby documentation project. Shuffle URL: http://ruby-doc.org/core-2.2.0/Array.html#method-i-shuffle [accessed 2016-02-17]
[WebCite Cache ID 6fNHpzEdI]
18. Google. Add parameters to URLs to identify the campaigns that refer traffic URL: https://support.google.com/analytics/
answer/1033863?hl=en [accessed 2016-02-17] [WebCite Cache ID 6fNHyqvir]
19. https://bitly.com/. Bitly URL: https://bitly.com/ [accessed 2016-02-17] [WebCite Cache ID 6fNHuGMkh]
20. https://buffer.com. Buffer URL: https://buffer.com [accessed 2016-02-17] [WebCite Cache ID 6fNI55AJe]
21. https://buffer.com/developers/api/updates. Buffer Rest API URL: https://buffer.com/developers/api/updates [accessed
2016-02-17] [WebCite Cache ID 6fNI81yL5]
22. Google. Google Analytics URL: https://www.google.com/analytics/ [accessed 2016-02-17] [WebCite Cache ID 6fNIDa8lO]
23. Creative Commons. MIT License URL: http://choosealicense.com/licenses/mit/ [accessed 2016-02-17] [WebCite Cache
ID 6fNH2emCR]
J Med Internet Res 2016 | vol. 18 | iss. 6 | e144 | p. 11http://www.jmir.org/2016/6/e144/
(page number not for citation purposes)
Reuter et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX

24. Utengen A. Symplur. 2012 Dec 10. The rise of patient communities on Twitter ? Twitter Visualized URL: http://www.
symplur.com/shorts/the-rise-of-patient-communities-on-twitter-visualized/#!prettyPhoto/0/ [accessed 2016-02-17] [WebCite
Cache ID 6fNGr1HmB]
25. Utengen A. Symplur. 2012 Dec 17. The Dynamics of a Twitter Patient Community – Network Centrality Analysis URL:
http://www.symplur.com/shorts/dynamics-twitter-patient-community-network-centrality-analysis/ [accessed 2016-02-17]
[WebCite Cache ID 6fNGh78Hu]
26. Google. Google Docs URL: https://www.google.com/docs/ [accessed 2016-02-17] [WebCite Cache ID 6fNGXzA6u]
27. Temkar P. Clinical operations generation next… The age of technology and outsourcing. Perspect Clin Res 2015;6(4):175-178
[FREE Full text] [doi: 10.4103/2229-3485.167098] [Medline: 26623386]
28. Garrett A, Brown Stafford P. Using Real-time Data to Drive Better Decisions, Faster. Therapeutic Innovation & Regulatory
Science 2011;45(4):495-502. [doi: 10.1177/009286151104500410]
29. Tufts University. 2013. Impact Report, Tufts Center for the Study of Drug Development URL: http://csdd.tufts.edu/files/
uploads/02_-_jan_15,_2013_-_recruitment-retention.pdf [accessed 2016-05-26] [WebCite Cache ID 6hn4grtTS]
30. Glassdoor. Social Media Manager Salaries URL: https://www.glassdoor.com/Salaries/
social-media-manager-salary-SRCH_KO0,20.htm [accessed 2016-02-17] [WebCite Cache ID 6fNGOy3Na]
31. Norah A, Yoo D, Mcdonald D. Dissecting a social botnet. In: CSCW. 2015 Presented at: Proceedings of the 18th ACM
Conference on Computer Supported Cooperative Work and Social Computing; March 14 - 18, 2015; Vancouver, BC,
Canada p. 839-851.
32. Tavares G, Faisal A. Scaling-laws of human broadcast communication enable distinction between human, corporate and
robot Twitter users. PLoS One 2013 Jul;8(7):e65774 [FREE Full text] [doi: 10.1371/journal.pone.0065774] [Medline:
23843945]
33. Hwang T, Pearce I, Nanis I. Socialbots: Voices from the fronts. Interactions 2012;19(2):38-45.
34. Kyumin L, Eoff B, Caverlee J. Seven months with the devils: A long-term study of content polluters on Twitter. In:
Association for the Advancement of Artificial Intelligence Digital Library. Menlo Park, California: The AAAI Press; 2011
Presented at: Proceedings of the 5th AAAI International Conference on Weblogs and Social media; July 17 – 21, 2011;
Barcelona, Catalonia, Spain.
35. Cook D, Waugh B, Abdipanah M, Hashemi O, Rahman S. Twitter deception and influence: Issues of identity, slacktivism,
and puppetry. Journal of Information Warfare 2014;13(1):58-71.
36. Eni M, Metaxas P. From obscurity to prominence in minutes: Political speech and real-time search. Raleigh, NC: US; 2010
Presented at: Proceedings of the WebSci10: Extending the Frontiers of Society On-Line; April 26-27, 2010; Raleigh, North
Carolina.
37. Yazan B, Muslukhov I, Beznosov K, Ripeanu M. The socialbot network: when bots socialize for fame and money. 2011
Presented at: 27th Annual Computer Security Applications Conference; December 05 - 09, 2011; Orlando, FL, USA p.
93-102.
38. Ratkiewicz J, Conover M, Meiss M, Gonçalves B, Flammini A, Menczer F. Detecting and tracking political abuse in social
media. In: Proceedings of the Fifth International AAAI Conference on Weblogs and Social Media. 2011 Presented at: 5th
International AAAI Conference on Weblogs and Social Media; July 17-21, 2011; Barcelona, Spain p. 297-304.
39. Maria A, Deplano M, Schifanella R, Ruffo G. People Are Strange When You’re a Stranger: Impact and Influence of Bots
on Social Networks. In: Proceedings of the Sixth International AAAI Conference on Weblogs and Social Media. Palo Alto,
California: The AAAI Press; 2012 Presented at: Sixth International AAAI Conference on Weblogs and Social Media; June
4–7, 2012; Dublin, Ireland p. 10-17.
40. Haustein S, Bowman T, Holmberg K, Tsou A, Sugimoto C, Larivière V. Tweets as impact indicators: Examining the
implications of automated “bot” accounts on Twitter. J Assn Inf Sci Tec 2015 May 05;67(1):232-238. [doi: 10.1002/asi.23456]
41. Wilkie A, Michael M, Plummer-Fernandez M. Speculative method and Twitter: Bots, energy and three conceptual characters.
Sociol Rev 2014 Jul 14;63(1):79-101. [doi: 10.1111/1467-954X.12168]
42. Lokot T, Diakopoulos N. News Bots. Automating news and information dissemination on Twitter. Digital Journalism 2015
Sep 15:1-18. [doi: 10.1080/21670811.2015.1081822]
43. Wagner C, Mitter S, Koerrner C, Strohmaier M. When social bots attack: Modeling susceptibility of users in online social
networks. New York: ACM; 2012 Presented at: Proceedings of the 2nd Workshop on Making Sense of Microposts
(MSM’2012), held in conjunction with the 21st World Wide Web Conference (WWW’2012); April 16 - 20, 2012; Lyon,
France.
44. Clerwall C. Enter the Robot Journalist. Journalism Practice 2014 Feb 25;8(5):519-531. [doi: 10.1080/17512786.2014.883116]
45. Edwards C, Edwards A, Spence P, Shelton A. Is that a bot running the social media feed? Testing the differences in
perceptions of communication quality for a human agent and a bot agent on Twitter. Computers in Human Behavior 2014
Apr;33:372-376. [doi: 10.1016/j.chb.2013.08.013]
46. Zarrella D. The science of marketing: When to tweet, what to post, how to blog, and other proven strategies. New York:
Wiley; 2013.
J Med Internet Res 2016 | vol. 18 | iss. 6 | e144 | p. 12http://www.jmir.org/2016/6/e144/
(page number not for citation purposes)
Reuter et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX

Abbreviations
API: application programming interface
CSV: comma-separated values
HIV: human immunodeficiency virus
ID: identifier
KPI: key performance indicator
LTS: long-term support
REST: representational state transfer
USC: University of Southern California
UTM: Urchin Traffic Monitor
Edited by G Eysenbach; submitted 17.02.16; peer-reviewed by M Bestek, I Brooks, J Teng; comments to author 18.03.16; revised
version received 07.04.16; accepted 24.04.16; published 29.06.16
Please cite as:
Reuter K, Ukpolo F, Ward E, Wilson ML, Angyan P
Trial Promoter: A Web-Based Tool for Boosting the Promotion of Clinical Research Through Social Media
J Med Internet Res 2016;18(6):e144
URL: http://www.jmir.org/2016/6/e144/
doi: 10.2196/jmir.4726
PMID: 27357424
©Katja Reuter, Francis Ukpolo, Edward Ward, Melissa L Wilson, Praveen Angyan. Originally published in the Journal of Medical
Internet Research (http://www.jmir.org), 29.06.2016. This is an open-access article distributed under the terms of the Creative
Commons Attribution License (http://creativecommons.org/licenses/by/2.0/), which permits unrestricted use, distribution, and
reproduction in any medium, provided the original work, first published in the Journal of Medical Internet Research, is properly
cited. The complete bibliographic information, a link to the original publication on http://www.jmir.org/, as well as this copyright
and license information must be included.
J Med Internet Res 2016 | vol. 18 | iss. 6 | e144 | p. 13http://www.jmir.org/2016/6/e144/
(page number not for citation purposes)
Reuter et alJOURNAL OF MEDICAL INTERNET RESEARCH
XSL
•
FO
RenderX
