
Wh o l e ef f lu e n t to x i c i t y • tr a i n in g Vi d e o Se r i e S • Saltwater Series
Sperm Cell Toxicity
Tests Using the Sea Urchin
(Arbacia punctulata)
Supplement to Training Video
U.S. Environmental Protection Agency
Ofce of Wastewater Management
Water Permits Division
1200 Pennsylvania Ave., NW
Washington, DC 20460
EPA 833-C-09-001
March 2009
NOTICE
The revision of this guide has been funded wholly or in part by the
Environmental Protection Agency under Contract EP-C-05-063. Mention of
trade names or commercial products does not constitute endorsement or
recommendation for use.

Sperm Cell Toxicity Tests Using the Sea Urchin (Arbacia punctulata)
Supplement to Training Video
i
U.S. ENVIRONMENTAL PROTECTION AGENCY
Foreword
This guide serves as a supplement to the video “Sperm Cell Toxicity Tests Using the Sea Urchin, Arbacia
punctulata” (EPA, 2009). The methods illustrated in the video and described in this supplemental guide
support the methods published in the U.S. Environmental Protection Agency’s (EPA’s) Short-term Methods
for Estimating the Chronic Toxicity of Efuents and Receiving Waters to Marine and Estuarine Organisms,
Third Edition (EPA, 2002a), referred to as the Saltwater Chronic Methods Manual. The video and this guide
provide details on preparing for and conducting the test based on the expertise of personnel at the follow-
ing EPA Ofce of Research and Development (ORD) laboratories:
National Health and Environmental Effects Research Laboratory (NHEERL) – Atlantic Ecology Division
in Narragansett, Rhode Island
NHEERL – Gulf Ecology Division in Gulf Breeze, Florida
National Exposure Research Lab (NERL) – Ecological Exposure Research Division (EERD) in
Cincinnati, Ohio
This guide and its accompanying video are part of a series of training videos produced by EPA’s Ofce of
Wastewater Management. This Saltwater Series includes the following videos and guides:
“Mysid (Americamysis bahia) Survival, Growth, and Fecundity Toxicity Tests”
“Culturing Americamysis bahia”
“Sperm Cell Toxicity Tests Using the Sea Urchin, Arbacia punctulata”
“Red Algal (Champia parvula) Sexual Reproduction Toxicity Tests”
“Sheepshead Minnow (Cyprinodon variegatus) and Inland Silverside (Menidia beryllina) Larval Survival
and Growth Toxicity Tests”
The Freshwater Series, released in 2006, includes the following videos and guides:
“Ceriodaphnia Survival and Reproduction Toxicity Tests”
“Culturing of Fathead Minnows (Pimephales promelas)”
“Fathead Minnow (Pimephales promelas) Larval Survival and Growth Toxicity Tests”
All of these videos are available through the National Service Center for Environmental Publications
(NSCEP) at 800 490-9198 or nscep@bps-lmit.com.

U.S. ENVIRONMENTAL PROTECTION AGENCY
ii
Sperm Cell Toxicity Tests Using the Sea Urchin (Arbacia punctulata)
Supplement to Training Video
Intentionally Left Blank

Sperm Cell Toxicity Tests Using the Sea Urchin (Arbacia punctulata)
Supplement to Training Video
iii
U.S. ENVIRONMENTAL PROTECTION AGENCY
Contents
Foreword ..........................................................................i
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Background . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Water and Light . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Obtaining and Maintaining Sea Urchins ................................................1
Culture Water . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Photoperiod . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1
Culture Vessels . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Water Delivery Systems .............................................................2
Food Preparation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Test Method . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Obtaining Gametes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Making Stock Solutions of Sperm and Eggs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
Efuent Preparation ................................................................5
Beginning the Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
Routine Chemistries ................................................................6
Terminating the Test ................................................................6
Test Acceptability and Data Review . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
Citations and Recommended References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
Glossary . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Glossary-1
Appendix A: Preparing Hypersaline Brine (HSB) .........................................A-1
Appendix B: Apparatus and Equipment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . B-1
Appendix C: Reagents and Consumable Materials . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . C-1
Appendix D: Summary of Test Conditions and Test Acceptability Criteria . . . . . . . . . . . . . . . . . . . D-1
Appendix E: Data Sheets ...........................................................E-1

U.S. ENVIRONMENTAL PROTECTION AGENCY
iv
Sperm Cell Toxicity Tests Using the Sea Urchin (Arbacia punctulata)
Supplement to Training Video
fi g u r e S
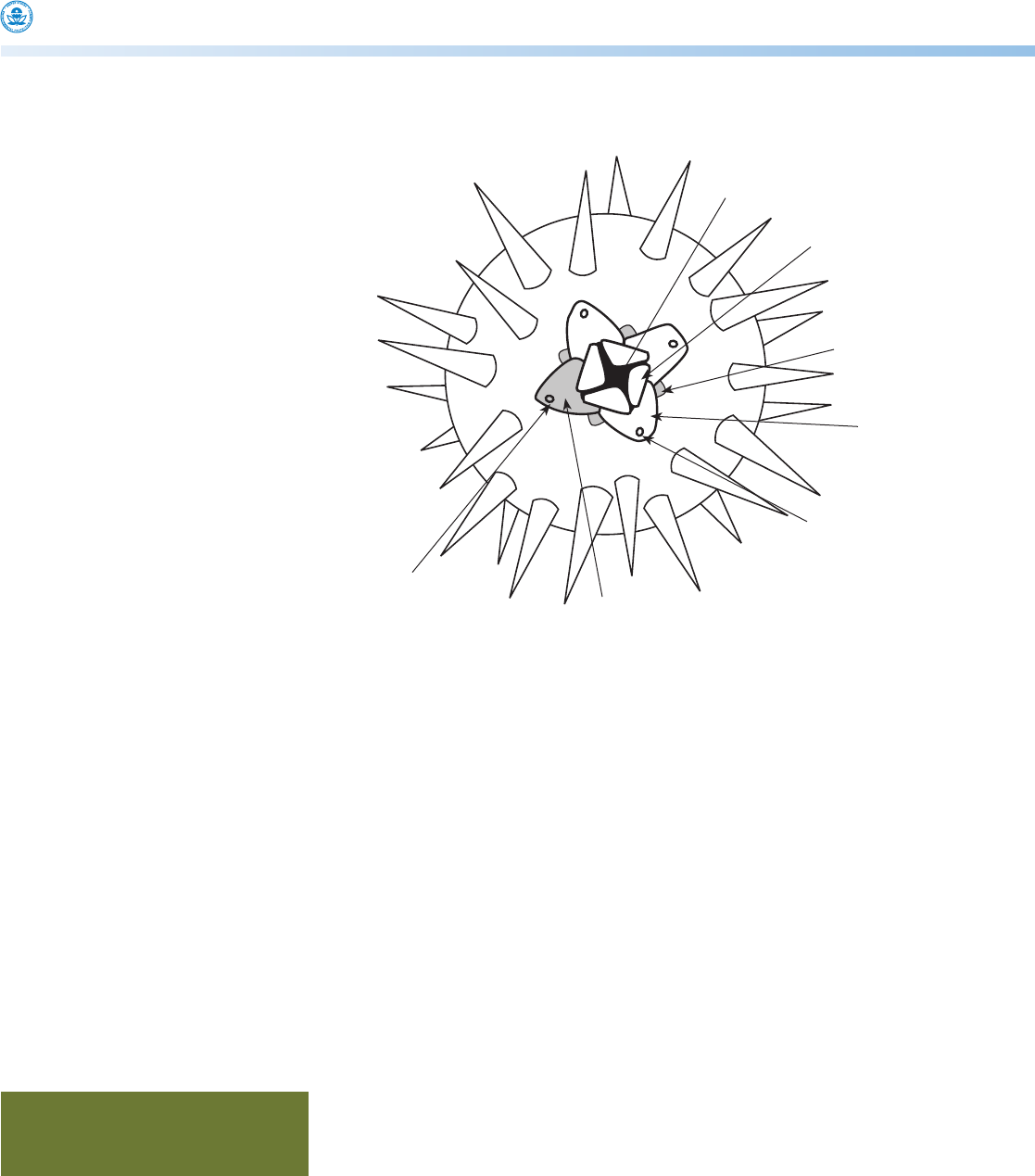
Figure 1. Schematic of the aboral surface of Arbacia punctulata, with spines partly removed to
show structure, especially the genital pores . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Figure E-1. Sperm Cell Toxicity Test, Sample Data Sheet #1 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .E-1
Figure E-2. Sperm Cell Toxicity Test, Sample Data Sheet #2 – Raw Data . . . . . . . . . . . . . . . . . . . .E-2
ta b l e S
Table 1. Fifty Percent Serial Dilution Method for Counting Sperm Cell Density. . . . . . . . . . . . . . . . . 3
Table A-1. Preparation of Test Solutions at a Salinity of 30‰ Using HSB for a Final Test
Concentration Volume of 1000 mL. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-2
Table A-2. Preparation of Test Solutions at a Salinity of 30‰ Using Natural Seawater, Hypersaline
Brine, or Articial Sea Salts ........................................................ A-2

Sperm Cell Toxicity Tests Using the Sea Urchin (Arbacia punctulata)
Supplement to Training Video
1
U.S. ENVIRONMENTAL PROTECTION AGENCY
Introduction
This supplemental guide accompanies the Environmental Protection Agency’s (EPA’s) video training for
conducting sea urchin (Arbacia punctulata) fertilization toxicity tests (EPA, 2009). The test method is found
in Section 15 of EPA’s Short-term Methods for Estimating the Chronic Toxicity of Efuents and Receiving
Waters to Marine and Estuarine Organisms, Third Edition (EPA, 2002a). The test was developed at EPA’s
Ofce of Research and Development’s (ORD’s) National Health and Environmental Effects Research
Laboratory-Atlantic Ecology Division (NHEERL-AED) in Narragansett, Rhode Island, and is based on the
freshwater tests developed at the EPA Mid-Continent Ecology Division (MED) in Duluth, Minnesota. The
material presented in both the video and this guide summarizes the methods but does not replace a thor-
ough review and understanding of the methods by laboratory personnel before conducting the test.
Background
Under the National Pollutant Discharge Elimination System (NPDES) program (Section 402 of the Clean
Water Act), EPA uses toxicity tests to monitor and evaluate efuents for their toxicity to biota and their
impact on receiving waters. By determining acceptable or safe concentrations for toxicants discharged
into receiving waters, EPA can establish NPDES permit limitations for toxicity. These WET (Whole efuent
toxicity) permit limitations regulate pollutant discharges on a whole efuent effect basis rather than by a
chemical-specic approach only.
Whole efuent toxicity methods measure the synergistic, antagonistic, and additive effects of all the chemi-
cal, physical, and additive components of an efuent that adversely affect the physiological and biochemi-
cal functions of the test organisms. Therefore, healthy organisms and correct laboratory procedures are
essential for valid test results. Laboratory personnel should be very familiar with the test methods and with
sea urchin handling techniques before conducting a test.
This supplemental guide covers the procedures for conducting the test according to EPA’s promulgated
methods (40 CFR Part 136; EPA, 2002c) and also provides some helpful information that is not presented
in the Saltwater Chronic Methods Manual (EPA, 2002a).
This test method examines the effect of efuent or receiving waters on the reproduction of sea urchin
gametes after exposure in a static system for 1 hour and 20 minutes. Sperm cells are exposed to a series
of efuent concentrations for 1 hour. The eggs are then introduced to the test chambers which contain
the sperm cells. After 20 minutes, the test is ended and the effects on exposed gametes are compared to
controls to determine if the efuent concentrations had any effect on fertilization.
This guide and the accompanying video describe how the test is set up, initiated, terminated, and reviewed,
including suggestions on maintaining healthy cultures of test animals.
Water and Light
obtaining a n d Maintaining Se a ur c h i n S
Before conducting tests, healthy sea urchin cultures should be established. Adult sea urchins can be
ordered from commercial biological supply houses, or collected along the Atlantic coast. Keep male and
female animals in separate tanks. To determine the sex of each animal, briey stimulate each with a
12-volt transformer. This causes the immediate release of masses of gametes from genital pores on the
top of the animal. The eggs are red and the sperm are white. Separate the animals into 20 L aerated ber-
glass tanks; each can hold about 20 adults.
U.S. ENVIRONMENTAL PROTECTION AGENCY
2
Sperm Cell Toxicity Tests Using the Sea Urchin (Arbacia punctulata)
Supplement to Training Video
cu lt u r e Wa t e r
The quality of water used for
maintaining sea urchins is very
important. Culture water and
all water used for washing and
dilution steps and for control
water in the tests should be
maintained at a salinity of
30‰ ± 2‰ using natural
seawater, hypersaline brine
(HSB) , or articial sea salts.
Instructions for making dilution
water and HSB are provided in
Appendix A of this document
and Section 7 of the Saltwater
Chronic Methods Manual (EPA,
2002a).
Ph o t o P e r i o d
The sea urchin conditions
should include a photoperiod
of 16 hours light and 8 hours
darkness. The light quality and
intensity should be at ambient laboratory levels, which is approximately 10 – 20 E/µm
2
/s or 50 to 100 foot
candles (ft-c) (EPA, 2002a).
cu lt u r e Ve S S e l S
Adult sea urchins are kept in natural or articial seawater in a ow-through or recirculating aerated 40-L
glass aquarium.
Allow ltered seawater to ow into the tanks at a rate of 5 L per minute and maintain the temperature at
15°C ± 3°C.
Wa t e r de l i V e r y Sy S t e M S
Equip the adult sea urchin aquarium with an under-gravel or outside biological lter, or cartridge lter. A
stock of at least 12 males and 12 females are needed for routine testing. If the animals will be used for an
on-site test, transport them separated by sex in separate or partitioned coolers packed with wet kelp and
paper towels. Once on site, the sea urchins should be transferred into separate 10-gallon aquarium tanks
with gravel-bed ltration. Even with ltration, the water should be changed periodically to maintain good
water quality.
fo o d Pr ePa r at i o n
Sea urchins are fed kelp of the species Laminaria obtained from uncon-
taminated coastal waters or ordered from commercial supply houses, or
romaine lettuce. Supply the urchins with ample food, renewing the kelp
each week and removing decaying kelp as necessary. Healthy sea urchins will attach to kelp or aquarium
walls within hours — any unhealthy animals should be removed and should not be used for testing. Every 1
to 2 weeks, empty and clean the tanks.
Collect eggs rst to avoid any
possible pre-fertilization.
Figure 1. Schematic of the aboral surface of Arbacia punctulata,
with spines partly removed to show structure, especially the genital
pores
madreporite
madreporic
plate
anus
suranal
plate
ocular
plate
genital
plate
genital
pore
Sperm Cell Toxicity Tests Using the Sea Urchin (Arbacia punctulata)
Supplement to Training Video
3
U.S. ENVIRONMENTAL PROTECTION AGENCY
Test Method
obtaining ga M e t e S
To prepare for the test, all vials, pipets, and pipet tips should be soaked in clean, 30‰ seawater overnight.
Collect eggs and sperm from healthy animals by transferring the animals into a shallow bowl lled with
enough control seawater to just cover their shells. Eggs are obtained from female sea urchins using electri-
cal stimulation by touching the shells close to the genital pores with
electrodes from a 10 – 12-volt transformer for about 30 seconds.
The red eggs pool on the sea urchin shell above the genital pores.
These are collected from the shell using a 10 mL disposable syringe
with an 18-gauge, blunt-tipped needle with the tip cut off so that it
will rest on the shell without puncturing it. After collection, the needle
is removed and the eggs emptied into conical centrifuge tubes. Pool the eggs and keep them at room tem-
perature until use, but not longer than a few hours. Four females should yield enough eggs to test ve test
dilutions plus one control, with four replicates.
Obtain sperm from four male sea urchins. Again, place the animals in a shallow bowl with their shells barely
covered with control seawater. Like the females, the males are induced to spawn by placing electrodes
from a 10 – 12-volt transformer against their shells for 30 seconds. The sperm appear white. Collect the
concentrated sperm that pools on top of the shell using a syringe tted with an 18-gauge, blunt-tipped
needle. Pool the sperm, keep the sample on ice, and record the collection time. The sperm must be used in
a toxicity test within 1 hour of collection.
Ma k i n g St o c k So l u t i o n S o f SP e r M a n d eg g S
To ensure reproducibility in the test results, the sperm and eggs must be concentrated to known dilutions
using the 30‰ seawater. During the exposure period, 2,500 sperm should be present for every one egg.
Figure E-1, presented in Appendix E, provides a sample data sheet used to calculate the sperm and egg
deliveries.
After collection, the sperm should be in a volume of about 0.5 to 1 mL of control water in the collecting
syringe. This is called the “sperm stock” solution. Perform a 50 percent serial dilution for counting the
sperm cell density using the following dilution method (see Table 1).
Add sperm from Vial E to both sides of a Neubauer hemacytometer. Let the sperm settle 15 minutes. Count
the number of sperm in the central 400 squares on both sides of the hemacytometer under a compound
microscope (100X).
The average of the two sperm cell counts (sperm/mL or SPM) from Vial E = # x 10
4
.
Calculate the SPM in all the other suspensions based on this count:
Vial A = 40 x SPM of Vial E
Vial B = 20 x SPM of Vial E
Vial D = 5 x SPM of Vial E
SPM of original sample = 2000 x SPM of Vial E
To prepare the sperm suspension for the test, select the vial containing an SPM greater than 5 x 10
7
SPM.
To determine the dilution needed for the test:
The calculated SPM
(5 x 10
7
)
= Dilution Factor (DF)
[(DF) x 10] - 10 = mL of seawater to add to selected vial
At AED, staff use the data
sheet included in Appendix E for
calculating and recording dilutions.
The egg solution can be prepared
during the rst hour of the test after
the sperm exposure has started.

U.S. ENVIRONMENTAL PROTECTION AGENCY
4
Sperm Cell Toxicity Tests Using the Sea Urchin (Arbacia punctulata)
Supplement to Training Video
The sperm cell count in the test stock should be conrmed. Add 0.1 mL of test stock to 9.9 mL of 10
percent acetic acid in seawater and count the sperm cells using a hemacytometer. This count
should average 50 ± 5 cells. Only about 2.5 mL of sperm test stock solution is
needed for testing 5 test solutions and a control, with 4 or more replicates. Hold the
test stock on ice until the test begins, but no longer than 1 hour.
The eggs must be washed before preparing the standard egg dilution needed for
the test (2,000 eggs/mL). To wash the eggs, rst remove the supernatant water
from the settled eggs. Add seawater and mix carefully by inversion. Spin the vial in a tabletop centrifuge at
the lowest possible setting (e.g., 500xg) for 3 minutes to form a lightly packed pellet. Wash and spin the
eggs twice more. If at any time the wash water appears red the eggs are lysing (the membranes have been
disturbed) and the eggs are unsuitable for testing; discard these eggs and start again.
After washing, transfer the washed eggs to a beaker containing 200 mL of control seawater. This is called
the “egg test stock.” Mix the stock solution using gentle aeration until the egg solution is homogenous. The
aeration device used in Narragansett is a 3-pronged diffuser attached by exible tubing to an air pump.
Vial A
20 mL stock
seawater
Vial B
10 mL stock
seawater
Vial C
10 mL stock
seawater
Vial D
10 mL stock
seawater
Vial C Vial E
4 mL stock
seawater
1. 400 µl
sperm stock
2. 10 ml
3. 10 ml
4. 10 ml 5. Discard 10 ml
6. 10 mL 10% acetic
acid in saltwater
7. 1 mL
Mix well
before each
transfer.
Table 1. Fifty Percent Serial Dilution Method for Counting Sperm Cell Density.
1. Add 400 µL of sperm stock to 20 mL of seawater to create Vial A. Mix by gently pipetting
using a 5-mL pipettor, or by inversion.
2. Add 10 mL from Vial A to 10 mL of seawater to create Vial B. Mix by gently pipetting using a
5-mL pipettor, or by inversion.
3. Add 10 mL from Vial B to 10 mL of seawater to create Vial C. Mix by gently pipetting using a
5-mL pipettor, or by inversion.
4. Add 10 mL from Vial C to 10 mL of seawater to create Vial D. Mix by gently pipetting using
a 5-mL pipettor, or by inversion.
5. Discard 10 mL from Vial D so that all vials now contain 10 mL.
6. Vial C is used to create a nal dilution that is killed and counted. Add 10 mL 10% acetic acid
in seawater to Vial C; cap the vial and mix by inversion.
7. Add 1 mL of the killed sperm in Vial C to 4 mL of seawater in Vial E. Mix by gently pipetting
using a 4-mL pipettor.

Sperm Cell Toxicity Tests Using the Sea Urchin (Arbacia punctulata)
Supplement to Training Video
5
U.S. ENVIRONMENTAL PROTECTION AGENCY
Make a 1:10 dilution of the test stock for the purpose of counting the eggs. Cut the point from a wide-
mouth pipet tip to make sure the eggs will not be damaged and transfer 1 mL of egg solution to a vial
containing 9 mL of control water. Mix by inversion.
Transfer 1 mL of the egg solution to a Sedgewick-Rafter counting chamber. Count the number of eggs
under a dissecting microscope at 25X magnication. Ten times the number of eggs in that milliliter equals
the number of eggs/mL in the egg stock. The target concentration for test initiation is 2,000 eggs/mL.
If the egg count is greater than or equal to 200 eggs, add the proper volume of water:
(# of eggs counted) - 200 = volume (mL) of control water to add
If less than 200 eggs were
counted, allow the eggs to
settle in the beaker, remove
the supernatant water to con-
centrate the eggs to greater
than 200, repeat the count,
and dilute the egg test stock
as described above.
Verify the concentration by
counting 1 mL of a 1:10
dilution of the adjusted stock
solution. The count for the
nal dilution should equal
100 ± 20 eggs/mL. The test
requires 24 mL of egg test
stock for a control and ve
exposure concentrations.
eff l ue n t Pr e Pa r at i o n
Efuent sampling should
be conducted according to
Section 8 of the Saltwater
Chronic Methods Manual
(EPA, 2002a) and any
specic requirements of a
NPDES permit. The efuent
or receiving waters should be
held at 0°C – 6°C until used
for testing. Under the NPDES
program, lapsed time from
sample collection to rst use in the test must not exceed 36 hours. Under special conditions or variances,
samples may be held longer but should never be used for testing if held for more than 72 hours.
Maintain the salinity of the test samples to 30‰ ± 2‰. To do this, efuent samples may need to be
adjusted using hypersaline brine (HSB). A recipe for HSB is provided in Appendix A of this manual.
Approximately 1 hour before the test is to begin, adjust approximately 1 L of efuent to the test tempera-
ture of 20ºC ± 1ºC and maintain that temperature while preparing the test concentrations. To test a series
of decreasing concentrations of efuent, use a dilution factor of ≥ 0.5. When starting with efuent that
has 0‰ salinity and using HSB, the maximum efuent concentration that can be prepared at 30‰ is 70
percent efuent. Table A-1 presents the volumes needed for the test concentrations using HSB.
Dilution Water
The type of dilution water used to make the test concentrations is
dependent on the objectives of the test. Any specic requirements
included in NPDES permits should be followed. The Saltwater Chronic
Methods Manual (Section 7) provides the following guidelines:
• If the test is conducted to estimate the absolute chronic toxicity
of the efuent, synthetic dilution water should be used. If the cultures
were maintained in different water than used for dilution water, a
second set of control replicates should be conducted using the culture
water.
• If the test is conducted to estimate the chronic toxicity of the
efuent in uncontaminated receiving waters, the test can be
conducted using a grab sample of the receiving waters collected outside
the inuence of the outfall, other uncontaminated waters, or standard
dilution water with the same salinity as the receiving waters. If the
cultures were maintained in different water than used for dilution water,
a second set of control replicates should be conducted using the culture
water.
• If the test is conducted to estimate the additive or mitigating
effects of the efuent on already contaminated receiving
waters, the test must be conducted using receiving waters collected
outside the inuence of the outfall. Controls should be conducted using
both receiving water and culture water.

U.S. ENVIRONMENTAL PROTECTION AGENCY
6
Sperm Cell Toxicity Tests Using the Sea Urchin (Arbacia punctulata)
Supplement to Training Video
be g i n n i n g t h e te S t
In Narragansett, disposable glass vials are used as test chambers. They are labeled with concentration
and replicate numbers and arranged in the partitioned cardboard box in which they are shipped. Prepare
the efuent dilutions for four replicates of each concentration and the control solution to reduce variability
among replicates. Each concentration should be prepared in one beaker and 5 mL distributed to each of the
test chambers. Be sure the efuent temperature has been brought up to 20ºC before beginning the test.
Within 1 hour of collecting and preparing the sperm test stock, add 100 µL of the well-mixed sperm test
stock to each test and control vial. Cover the chambers, record the time, and maintain the chambers at
20ºC ± 1ºC for 1 hour.
At the end of the hour, mix the egg test stock using gentle aeration and add 1 mL of the egg solution to
each exposure vial using a wide-mouth pipet. When all of the vials contain eggs, lift the storage box and
gently move it in circles to “swirl” the egg-sperm suspension. Cover the chambers, record the time, and
incubate the eggs and sperm at 20ºC ± 1ºC for 20 minutes.
ro u t i n e ch e M i S t r i e S
At the beginning of the exposure period, DO, pH, temperature and salinity are measured in one chamber at
each test concentration and the control.
terMinating t h e te S t
After 20 minutes, end the test and preserve the samples by adding 2 mL of 1% formalin in seawater to
each vial. Cap the vials and record the time. The test should be evaluated immediately but can be evalu-
ated up to 48 hours later.
Test Acceptability and Data Review
This test demonstrates the efuent or receiving water’s effect on sea urchin fertilization. To evaluate this,
exposed and control eggs are examined under a microscope and the number of unfertilized eggs in each
test chamber is recorded.
For each replicate, transfer about 80 – 120 µL of the preserved eggs to a multiple-chamber counting slide.
If a Sedgewick-Rafter counting chamber is used, transfer about 1 mL. Using a compound microscope at
100X magnication, observe 100 – 200 eggs per sample. This should be done with adequate ventilation,
preferably under a hood, to reduce exposure to the formalin fumes.
For each test chamber, record the total number of eggs counted, and the number that were not fertilized.
Fertilized eggs are surrounded by a fertilization membrane, while unfertilized eggs lack this membrane.
Abnormal eggs are not counted. Figure E-2 in Appendix E provides a sample data collection sheet.
Test data are reviewed to verify that test acceptability criteria (TAC) requirements for a valid test have been
met. For the test to be acceptable, the control chambers are required to have between 70% and 90% fer-
tilization of the eggs. The concentration-response relationship generated for each multi-concentration test
must be reviewed to ensure that calculated test results are interpreted appropriately. In conjunction with
this requirement, EPA has provided recommended guidance for concentration-response relationship review
(EPA, 2000b).
EPA’s promulgated toxicity testing method manuals (EPA, 2002a, b) recommend the use of point estima-
tion technique approaches for calculating endpoints for efuent toxicity tests under the NPDES program.
The promulgated methods also require a data review of toxicity data and concentration-response data, and
require calculating the percent minimum signicant difference (PMSD) when point estimation (e.g., LC
50
,
IC
25
) analyses are not used. EPA species the PMSD must be calculated when NPDES permits require sub-
lethal hypothesis testing. EPA also requires that variability criteria be applied as a test review step when
6

Sperm Cell Toxicity Tests Using the Sea Urchin (Arbacia punctulata)
Supplement to Training Video
7
U.S. ENVIRONMENTAL PROTECTION AGENCY
NPDES permits require sub-lethal hypothesis testing endpoints (i.e., no observed effect concentration
[NOEC] or lowest observed effect concentration [LOEC]) and the efuent has been determined to have no
toxicity at the permitted receiving water concentration (EPA, 2002b). This reduces the within-test variabil-
ity and increases statistical sensitivity when test endpoints are expressed using hypothesis testing rather
than the preferred point estimation techniques.
The sea urchin sperm cell test is currently used to assess the potential toxic effects of complex chemical
mixtures on marine and estuarine organisms. Used in conjunction with chemical-specic methods, this test
can provide a comprehensive and effective approach to assessing the impact of complex efuents dis-
charged to the marine and estuarine environments.
Citations and Recommended References
EPA. 1991. Technical Support Document for Water Quality-based Toxics Control. U.S. EPA Ofce of Water
Enforcement and Permits, Washington, D.C. EPA-505-2-90-001.
EPA. 2000a. Method Guidance and Recommendations for Whole Efuent Toxicity (WET) Testing (40 CFR
Part 136). Ofce of Water, Washington, D.C. EPA 821-B-00-004.
EPA. 2000b. Understanding and Accounting for Method Variability in Whole Efuent Toxicity Applications
Under the National Pollutant Discharge Elimination System Program. Ofce of Wastewater
Management, Washington, D.C. EPA 833-R-00-003.
EPA. 2002a. Short-term Methods for Estimating the Chronic Toxicity of Efuents and Receiving Waters to
Marine and Estuarine Organisms, Third Edition. (Saltwater Chronic Methods Manual). Ofce of
Water, Cincinnati, OH. EPA-821-R-02-014.
EPA. 2002b. Methods for Measuring the Acute Toxicity of Efuents and Receiving Waters to Freshwater
and Marine Organisms, Fifth Edition. (Acute Methods Manual). Ofce of Water, Cincinnati, OH.
EPA-821-R-02-012.
EPA. 2002c. Final Rule. 40 CFR Part 136. Guidelines Establishing Test Procedures for the Analysis of
Pollutants; Whole Efuent Toxicity Test Methods. 67 FR 69952-69972, November 19, 2002.
EPA. 2009. Sperm Cell Toxicity Tests Using the Sea Urchin (Arbacia punctulata). Supplement to Training
Video. Whole Efuent Toxicity Training Video Series, Saltwater Series. March 2009. EPA 833-C-
09-001.
EPA references are available online at www.epa.gov/npdes.
If you need additional copies of this document, you can download it at:
www.epa.gov/npdes/wqbasedpermitting.

U.S. ENVIRONMENTAL PROTECTION AGENCY
8
Sperm Cell Toxicity Tests Using the Sea Urchin (Arbacia punctulata)
Supplement to Training Video
Intentionally Left Blank

Glossary-1
U.S. ENVIRONMENTAL PROTECTION AGENCY Sperm Cell Toxicity Tests Using the Sea Urchin (Arbacia punctulata)
Supplement to Training Video
Glossary
Acute toxicity. An adverse effect measured on a group of test organisms during a short-term exposure in
a short period of time (96 hours or less in toxicity tests). The effect can be measured in lethality
or any variety of effects.
Arbacia punctulata. A species of Arbacia genus of purple-spined sea urchins. Its natural habitat is in the
Western Atlantic Ocean. Arbacia punctulata can be found in shallow water from Massachusetts
to Cuba and the Yucatan Peninsula, from Texas to Florida in the Gulf of Mexico, the coast from
Panama to French Guiana and in the Lesser Antilles, usually on rocky, sandy, or shelly bottoms.
Chronic toxicity. An adverse effect that occurs over a long exposure period. The effect can be lethality,
impaired growth, reduced reproduction, etc.
Diluent water. Dilution water used to prepare the efuent concentrations.
Efuent concentrations. Concentrations or dilutions of an efuent sample to which test organisms are
exposed to determine the biological effects of the sample on the test organism.
Efuent sample. A representative collection of the discharge that is to be tested.
Flow-through water delivery system. An open water ow system that delivers fresh water or seawater to
culture tanks and is disposed of after it leaves those tanks.
Hypothesis testing. Technique (e.g., Dunnett’s test) that determines what concentration is statistically
different from the control. Endpoints determined from hypothesis testing are NOEC and LOEC.
IC
25
(Inhibition Concentration, 25%). The point estimate of the toxicant concentration that would cause a
25% reduction in a non-quantal biological measurement (e.g., reproduction or growth) calculated
from a continuous model.
Laminaria. The scientic name for a species of kelp given as food to laboratory sea urchins.
LC
50
(Lethal Concentration, 50%). The concentration of toxicant or efuent that would cause death to
50% of the test organisms at a specic time of observations (e.g., 96-hour LC
50
).
Lowest Observed Effect Concentration (LOEC). The LOEC is the lowest concentration of toxicant to
which organisms are exposed in a test, which causes statistically signicant adverse effects on
the test organisms (i.e., where the values for the observed endpoints are statistically signicantly
different from the control). The denitions of NOEC and LOEC assume a strict dose-response
relationship between toxicant concentration and organism response.
Minimum Signicant Difference (MSD). The MSD is the magnitude of difference from the control where
the null hypothesis is rejected in a statistical test comparing a treatment with a control. MSD
is based on the number of replicates, control performance and power of the test. MSD is often
measured as a percent and referred to as PMSD.
No Observed Effect Concentration (NOEC). The NOEC is the highest tested concentration of toxicant to
which organisms are exposed in a full life-cycle or partial life-cycle (short-term) test, that causes
no observable adverse effect on the test organism (i.e., the highest concentration of toxicant
at which the values for the observed responses are not statistically signicantly different from
the controls). NOECs calculated by hypothesis testing are dependent upon the concentrations
selected.

U.S. ENVIRONMENTAL PROTECTION AGENCY
Glossary-2
Sperm Cell Toxicity Tests Using the Sea Urchin (Arbacia punctulata)
Supplement to Training Video
NPDES (National Pollutant Discharge Elimination System) Program. The national program for issuing,
modifying, revoking, and reissuing, terminating, monitoring and enforcing permits, and imposing
and enforcing pretreatment requirements, under Sections 307, 318, 402, and 405 of the Clean
Water Act.
Point Estimation Techniques. This technique is used to determine the efuent concentration at which
adverse effects (e.g., fertilization, growth or survival) occurred, such as Probit, Interpolation
Method, Spearman-Karber. For example, a concentration at which a 25% reduction in
reproduction and survival occurred.
Receiving Water Concentration (RWC). The RWC is the concentration of a toxicant or the parameter
toxicity in the receiving water (i.e., riverine, lake, reservoir, estuary or ocean) after mixing.
Recirculating water delivery system. A water ow system that treats water after it passes through the
culture tanks (usually with sand and biolters) and delivers the same treated water back to the
tanks.
Toxicity test. A procedure to measure the toxicity of a chemical or efuent using living organisms. The
test measures the degree of response of an exposed organism to a specic chemical or efuent.
WET (Whole efuent toxicity). The total toxic effect of an efuent measured directly with a toxicity test.

Sperm Cell Toxicity Tests Using the Sea Urchin (Arbacia punctulata)
Supplement to Training Video
A-1
U.S. ENVIRONMENTAL PROTECTION AGENCY
Appendix A:
Preparing Hypersaline Brine (HSB)
Salinity adjustments are a vital part of using marine and estuarine species for toxicity testing. Because the
majority of industrial and sewage treatment efuents entering marine and estuarine waters contain little or
no measurable salts, the salinity of these efuents must be adjusted before exposing estuarine or marine
plants and animals to the test solutions. It also is important to maintain constant salinity across all treat-
ments throughout the test for quality control. Finally, matching the test solution’s salinity to the expected
receiving water’s salinity may require salinity adjustments. NHEERL-AED uses HSB, prepared from ltered
natural seawater, to adjust exposure solution salinities.
HSB has several advantages over articial sea salts that make it more suitable for use in toxicity testing.
Concentrated brine derived from natural seawater contains the necessary trace metals, biogenic colloids,
and some of the microbial components necessary for adequate growth, survival, and/or reproduction of
test organisms. HSB can be held for prolonged periods without any apparent degradation, added directly to
the efuent to increase the salinity, or used as control water by diluting to the desired salinity with deion-
ized water. The brine can be made from any high quality, ltered seawater supply through simple heating
and aerating.
ge n e r at i n g t h e br i n e
The ideal container for making brine from natural seawater has a high surface-to-volume ratio, is made of a
non-corrosive material, and is easily cleaned. Shallow berglass tanks are ideal.
Thoroughly clean the tank, aeration supply tube, heater, and any other materials that will be in direct
contact with the brine before adding seawater to the tank. Use a good quality biodegradable detergent, fol-
lowed by several thorough deionized-water rinses.
Collect high-quality (and preferably high-salinity) seawater on an incoming tide to minimize the possibility of
contamination. Special care should be used to prevent any toxic materials from coming in contact with the
seawater. The water should be ltered to at least 10 µm before placing into the brine tank. Fill the tank with
seawater, and slowly increase the temperature to 40°C. If a heater is immersed directly into the seawater, make
sure that the heater components will not corrode or leach any substances that could contaminate the brine. A
thermostatically controlled heat exchanger made from berglass is suggested.
Aeration prevents temperature stratication and increases the rate of evaporation. Use an oil-free air
compressor to prevent contamination. Evaporate the water for several days, checking daily (or more or
less often, depending on the volume being generated) to ensure that the salinity does not exceed 100‰
and the temperature does not exceed 40°C. If these changes are exceeded, irreversible changes in the
brine’s properties may occur. One such change noted in original studies at NHEERL-AED was a reduction
in the alkalinity of seawater made from brine with salinity greater than 100‰, and a resulting reduction in
the animals’ general health. Additional seawater may be added to the brine to produce the volume of brine
desired.
When the desired volume and salinity of brine is prepared, lter the brine through a 1-mm lter and pump
or pour it directly into portable containers (20-L cubitainers or polycarbonate water cooler jugs are most
suitable). Cap the containers, and record the measured salinity and the date generated. Store the brine in
the dark at room temperature.

U.S. ENVIRONMENTAL PROTECTION AGENCY
A-2
Sperm Cell Toxicity Tests Using the Sea Urchin (Arbacia punctulata)
Supplement to Training Video
Sa l i n i t y ad j u S t M e n t S uS i n g hy P e r S a l i n e br i n e
To calculate the volume of brine (V
b
) to add to a 0‰ sample to produce a solution at a desired salinity (S
f
),
use this equation:
V
b
* S
b
= S
f
* V
f
Where: V
b
= volume of brine, mL
S
b
= salinity of brine, ‰
S
f
= nal salinity, ‰
V
f
= nal volume needed, mL
Table A-1 presents volumes needed to make 30‰ test solutions from efuent (0‰), deionized water, and
100‰ HSB. At 30‰ salinity, the highest achievable concentration is 70% efuent.
Table A-1. Preparation of Test Solutions at a Salinity of 30‰ Using HSB for a Final Test Concentration
Volume of 1000 mL.
Exposure
Concentration (%)
Efuent
(0 ‰)
(mL)
Deionized Water
(mL)
Hypersaline Brine
(100 ‰)
(mL)
70 700 — 300
25 250 450 300
7 70 630 300
2.5 25 675 300
0.7 7 693 300
Control — 1,000 —
Table A-2 gives examples of attainable exposure concentrations and dilution volumes needed when an
efuent salinity is raised to 30‰ using articial sea salts and using 0.5 serial dilution.
Table A-2. Preparation of Test Solutions at a Salinity of 30‰ Using Natural Seawater or Articial Sea Salts.
1
Solutions To Be Combined
Efuent Solution Efuent Concentration
(%)
Volume of Efuent
Solution (mL)
Volume of Diluent
Seawater (30‰) (mL)
1 100 840 —
2 50 420 Solution 1 + 420
3 25 420 Solution 2 + 420
4 12.5 420 Solution 3 + 420
5 6.25 420 Solution 4 + 420
Control 0.0 420
Total 2,080
1
This illustration assumes: 1) the use of 5 mL of test solution in each of four replicates (total of 20 mL) for the control and ve concentra-
tions of efuent, 2) an efuent dilution factor of 0.5, 3) the efuent lacks appreciable salinity, and 4) 400 mL of each test concentration is
used for chemical analysis. A sufcient initial volume (840 mL) of efuent is prepared by adjusting the salinity to 30‰. In this example, the
salinity is adjusted by adding articial sea salts to the 100% efuent, and preparing a serial dilution using 30‰ seawater (natural seawater,
HSB, or articial seawater). Stir solutions 1 hour to ensure that the salts dissolve. The salinity of the initial 840 mL of 100% efuent is
adjusted to 30‰ by adding 25.2 g of dry articial sea salts (FORTY FATHOMS®). Test concentrations are then made by mixing appropri-
ate volumes of salinity adjusted efuent and 30‰ salinity dilution water to provide 840 mL of solution for each concentration. If HSB alone
(100‰) is used to adjust the salinity of the efuent, the highest concentration of efuent that could be tested would be 70% at 30‰ salinity.
Source: EPA, 2002a.

Sperm Cell Toxicity Tests Using the Sea Urchin (Arbacia punctulata)
Supplement to Training Video
B-1
U.S. ENVIRONMENTAL PROTECTION AGENCY
Appendix B:
Apparatus and Equipment
Air lines, and air stones. For aerating water containing adults, or for supplying air to test solutions with
low DO.
Air pump. For oil-free air supply.
Balance. Analytical, capable of accurately weighing to 0.00001 g.
Beakers or asks. Six, borosilicate glass or non-toxic plasticware, 1000 mL for making test solutions.
Centrifuge. Bench-top, slant-head, variable speed for washing eggs.
Centrifuge tubes. Conical for washing eggs.
Compound microscope. For examining and counting sperm cells and fertilized eggs (25X and 100X).
Count register. 2-place for recording sperm and egg counts.
Cylindrical glass vessel. 8-cm diameter for maintaining dispersed egg suspension.
Dissecting microscope. For counting diluted egg stock (100X).
Environmental chamber or equivalent facility with temperature control (20°C ± 1°C).
Fume hood. To protect from formaldehyde fumes.
Glass dishes. Flat bottomed, 20-cm diameter for holding sea urchins during gamete collection.
Hemacytometer, Neubauer. For counting sperm.
Ice bucket. Covered for maintaining live sperm after collection until test initiation.
Laboratory sea urchins, Arbacia punctulata, culture unit. To test efuent or receiving water toxicity,
sufcient eggs and sperm must be available from healthy adult animals.
Meters: pH and DO, and specic conductivity. For routine physical and chemical measurements.
Pipets, automatic. Adjustable 1 – 100 mL.
Pipets, serological. 1 – 10 mL, graduated.
Pipets, volumetric. Class A, 1 – 100 mL.
Pipet bulbs and lters. Propipet
®
, or equivalent.
Reference weights, Class S. For checking performance of balance. Weights should bracket the expected
weights of materials to be weighed.
Refractometer or other method. For determining salinity.
Samplers. Automatic sampler, preferably with sample cooling capability, that can collect a 24-hour
composite sample of 5 L.

U.S. ENVIRONMENTAL PROTECTION AGENCY
B-2
Sperm Cell Toxicity Tests Using the Sea Urchin (Arbacia punctulata)
Supplement to Training Video
Sedgwick-Rafter counting chamber. For counting egg stock and examining fertilized eggs.
Syringes. 1 mL, and 10 mL, with 18 gauge, blunt-tipped needles (tips cut off) for collecting sperm and
eggs.
Thermometers. National Bureau of Standards Certied (see EPA 2002a). Used to calibrate laboratory
thermometers.
Thermometers, glass or electronic, laboratory grade. For measuring water temperatures.
Transformer, 10–12 Volt. With steel electrodes for stimulating release of eggs and sperm.
Vacuum suction device. For washing eggs.
Volumetric asks and graduated cylinders. Class A, Borosilicate glass or non-toxic plastic labware,
10 – 1000 mL for making test solutions.
Wash bottles. For deionized water, for washing organisms from containers and for rinsing small glassware
and instrument electrodes and probes.
Water purication system. Millipore
®
Milli-Q
®
deionized water or equivalent.

Sperm Cell Toxicity Tests Using the Sea Urchin (Arbacia punctulata)
Supplement to Training Video
C-1
U.S. ENVIRONMENTAL PROTECTION AGENCY
Appendix C:
Reagents and Consumable Materials
Acetic acid. 10%, reagent grade, in seawater for preparing killed sperm dilutions.
Buffers pH 4, pH 7, and pH 10. (Or as per instructions of instrument manufacturer) for standards and
calibration check.
Data sheets (one set per test). For data recording (see Appendix E).
Efuent, receiving water, and dilution water. Test waters, including efuent, receiving, and dilution
water should be analyzed to ensure its quality prior to using in tests. Dilution water containing
organisms that might prey upon or otherwise interfere with the test organisms should be ltered
through a ne mesh (with 150 µm or smaller openings).
Food. Kelp, Laminaria sp., or romaine lettuce for the sea urchin, Arbacia punctulata.
Formalin. 1%, in 2 mL of seawater for preserving eggs at end of test.
Gloves, disposable; lab coat and protective eyewear. For personal protection from contamination.
Laboratory quality assurance samples and standards. For calibration of the above methods.
Markers, waterproof. For marking containers, etc.
Paralm. To cover tubes and vessels containing test materials.
Reagent water Distilled or deionized water that does not contain substances which are toxic to the test
organisms.
Reference toxicant solutions. Reference toxicants such as sodium chloride (NaCl), potassium chloride
(KCl), cadmium chloride (CdCl
2
), copper sulfate (CuSO
4
), sodium dodecyl sulfate (SDS), and
potassium dichromate (K
2
Cr
2
O
7
), are suitable for use in the NPDES Program and other Agency
programs requiring aquatic toxicity tests.
Saline test and dilution water. The salinity of the test water must be in the range of 20‰ – 30‰. The
salinity should vary by no more than ± 2‰ among the chambers on a given day. If efuent and
receiving water tests are conducted concurrently, the salinities of these tests should be similar.
It is important to maintain a constant salinity across all treatments during a test. It is desirable
to match the test salinity with that of the receiving water. Two methods are available to adjust
salinities — a hypersaline brine (HSB) derived from natural seawater or articial sea salts. Both
are described in EPA, 2002.
Sample containers. For sample shipment and storage.
Sea Urchins. Arbacia punctulata, minimum of 12 of each sex.
Scintillation vials. 20 mL, disposable; to prepare test concentrations.
Standard salt water aquarium or Instant Ocean Aquarium. Capable of maintaining seawater at 15°C,
with appropriate ltration and aeration system.
Tape, colored. For labeling tubes.

U.S. ENVIRONMENTAL PROTECTION AGENCY
C-2
Sperm Cell Toxicity Tests Using the Sea Urchin (Arbacia punctulata)
Supplement to Training Video
Intentionally Left Blank

Sperm Cell Toxicity Tests Using the Sea Urchin (Arbacia punctulata)
Supplement to Training Video
D-1
U.S. ENVIRONMENTAL PROTECTION AGENCY
Appendix D:
Summary of Test Conditions and Test Acceptability
Criteria
Summary of Test Conditions and Test Acceptability Criteria for Sea Urchin, Arbacia punctulata, Fertilization Test with Efuent and
Receiving Waters (Test Method 1008.0)
1
Test type Static, non-renewal (required)
Salinity
30‰ ± 2‰ of the selected test salinity (recommended)
Temperature (Cº) 20°C ± 1ºC (recommended) Test temperatures must not deviate by more
than 3ºC during the test (i.e., max. temp – min. temp ≤ 3ºC) (required)
Light quality Ambient laboratory light during test preparation (recommended)
Light intensity 10 – 20 µE/m
2
/s, or 50 – 100 ft-c (Ambient laboratory levels) (recommend-
ed)
Test chamber size Disposable (glass) liquid scintillation vials (20 mL capacity), pre-soaked in
control water (recommended)
Test solution volume 5 mL (recommended)
Number of sea urchins Pooled eggs from 4 females and pooled sperm from 4 males per test
(recommended)
Number of eggs and sperm cells per chamber About 2,000 eggs and 5,000,000 sperm cells per vial (recommended)
Number of replicate chambers per concentration 4 (required minimum)
Dilution water Uncontaminated source of natural seawater; deionized water mixed with
HSB or articial sea salts (available options)
Test concentrations Efuents: 5 and a control (required minimum) Receiving waters: 100%
receiving water (or minimum of 5) and a control (recommended)
Dilution factor Efuents: ≥ 0.5 (recommended)
Receiving Waters: None or ≥ 0.5 (recommended)
Test duration 1 hour and 20 minutes (required)
Endpoint Fertilization of sea urchin eggs (required)
Test acceptability criteria 70% – 90% egg fertilization in controls (required)
Sampling requirements For on-site tests, one sample collected at test initiation, and used within
24 hr of the time it is removed from the sampling device. For off-site tests,
holding time must not exceed 36 hr before rst use for NPDES compliance
testing. (required)
Sample volume required 1 L per test (recommended)
1
Source: EPA, 2002a. For the purposes of reviewing WET test data submitted under NPDES permits, each test condition
listed above is identied as required or recommended. Additional requirements may be provided in individual permits,
such as specifying a given test condition where several options are given in the method.

U.S. ENVIRONMENTAL PROTECTION AGENCY
D-2
Sperm Cell Toxicity Tests Using the Sea Urchin (Arbacia punctulata)
Supplement to Training Video
Intentionally Left Blank

Sperm Cell Toxicity Tests Using the Sea Urchin (Arbacia punctulata)
Supplement to Training Video
E-1
U.S. ENVIRONMENTAL PROTECTION AGENCY
Appendix E:
Data Sheets
Figure E-1. Sperm Cell Toxicity Test, Sample Data Sheet #1
Test ID: ____________________________
Performed By: _______________________
Sperm Dilutions:
Hemacytometer Count, E: ________ X 10
4
= SPM “E”
Sperm Concentrations “E” X 40 = A = _______ SPM
“E” X 20 = B = _______ SPM
“E” X 5 = D = _______ SPM
Solution Selected for Test (> 5 X 10
7
SPM): ________
Dilution: SPM/(5 X 10
7
) = ________ DF
((DF) X 10) - 10 = _________ + SW, mL
Final Sperm Counts = _________
Egg Dilutions:
Initial Egg Count: = _________
Egg Stock Concentration = Egg Count (1 mL of 1:10 dilution) X 10: = _________
(Allow eggs to resettle and recount until count ≤ 200)
Volume of SW to Add to Dilute Egg Stock to 2000/mL: Egg Count – 200: = _______
Verify Final Egg Count (in 1 mL of 1:10 dilution): = _________
(Count should = 100 ± 20 eggs/mL)
Test Stocks:
Sperm Stock: ________ (5 X 10
7
SPM)
Volume Added/Test Vial: ________ (100 µL)
Egg Stock: ________ (2000/mL)
Volume Added/Test Vial ________ (1 mL)
Test Times:
Sperm Collection: ________
Egg Collection: ________
Sperm Added: ________
Eggs Added: ________
Fixative Added: ________
Samples Read: ________
Salinities:

U.S. ENVIRONMENTAL PROTECTION AGENCY
E-2
Sperm Cell Toxicity Tests Using the Sea Urchin (Arbacia punctulata)
Supplement to Training Video
Figure E-2. Sperm Cell Toxicity Test, Sample Data Sheet #2 – Raw Data
Test ID: ____________________________ Time: _______________
Performed by: _____________________ Date: _______________
Egg Counts at End of Test
Replicate 1 Replicate 2 Replicate 3 Replicate 4
Conc. (%) Total Unfert Tot al Unfert Total Unfert Total Unfert
Statistical Analysis:
Analysis of variance: _______________________________________________________________
Control: _______________________________________________________________
_______________________________________________________________
Different from Control (P): _______________________________________________________________
_______________________________________________________________
Comments: _______________________________________________________________
_______________________________________________________________
_______________________________________________________________
_______________________________________________________________
_______________________________________________________________
v
If you need additional copies of this document, you can download it at:
www.epa.gov/npdes/wqbasedpermitting
