G:\4177dft.doc
Guidance for Industry
Postmarketing Safety Reporting
for Human Drug and Biological
Products Including Vaccines
DRAFT GUIDANCE
This guidance document is being distributed for comment purposes only.
Comments and suggestions regarding this draft document should be submitted within 60
days of publication in the Federal Register of the notice announcing the availability of the
draft guidance. Submit comments to Dockets Management Branch (HFA-305), Food and
Drug Administration, 5630 Fishers Lane, rm. 1061, Rockville, MD 20857. All comments
should be identified with the docket number listed in the notice of availability that publishes
in the Federal Register.
For questions on the content of the draft document contact (CDER) Min Chen, 301-827-
3169 (phone); 301-827-5190 (fax), or (CBER) Miles Braun, 301-827-3974 (phone); 301-
827-3529 (fax).
U.S. Department of Health and Human Services
Food and Drug Administration
Center for Drug Evaluation and Research (CDER)
Center for Biologics Evaluation and Research (CBER)
March 2001
G:\4177dft.doc
Guidance for Industry
Postmarketing Safety Reporting
for Human Drug and Biological
Products Including Vaccines
Additional copies are available from:
Drug Information Branch (HFD-210)
Center for Drug Evaluation and Research (CDER)
5600 Fishers Lane, Rockville, MD 20857
(Tel) 301-827-4570
Internet at http://www.fda.gov/cder/guidance/index.htm
or
Office of Communication, Training and Manufacturers Assistance (HFM-40)
Center for Biologics Evaluation and Research (CBER)
1401 Rockville Pike, Rockville, MD 20852-1448
(Fax) 1-888-CBERFAX or 301-827-3844
(Voice Information) 1-800-835-4709 or 301-827-1800
Internet at http://www.fda.gov/cber/guidelines.htm
U.S. Department of Health and Human Services
Food and Drug Administration
Center for Drug Evaluation and Research (CDER)
Center for Biologics Evaluation and Research (CBER)
March 2001
G:\4177DFT.DOC
TABLE OF CONTENTS
I. INTRODUCTION..................................................................................................................................................................1
A. WHAT DOES THIS GUIDANCE DISCUSS?..........................................................................................................................1
B. WHAT DOES THIS GUIDANCE NOT DISCUSS?.................................................................................................................2
C. GOOD GUIDANCE PRACTICES.............................................................................................................................................2
II. BACKGROUND....................................................................................................................................................................3
A. FINAL RULES.......................................................................................................................................................................3
B. GUIDANCES .........................................................................................................................................................................3
C. PROPOSED RULES ...............................................................................................................................................................4
III. WHO MUST REPORT.........................................................................................................................................................4
IV. WHAT DO I REPORT?.......................................................................................................................................................5
A. TYPE OF ADVERSE EXPERIENCES .....................................................................................................................................6
B. DATA ELEMENTS TO INCLUDE IN A POSTMARKETING INDIVIDUAL CASE SAFETY REPORT .........................................8
V. TYPE OF REPORTS ............................................................................................................................................................9
A. 15-DAY REPORTS OF SERIOUS, UNEXPECTED ADVERSE EXPERIENCES........................................................................9
B. PERIODIC REPORTS ..........................................................................................................................................................11
C. FOLLOWUP REPORTS .......................................................................................................................................................15
D. DISTRIBUTION REPORTS FOR BIOLOGICAL PRODUCTS INCLUDING VACCINES ............................................................18
VI. SPECIAL REPORTING SITUATIONS .....................................................................................................................18
A. SCIENTIFIC LITERATURE REPORTS.................................................................................................................................18
B. POSTMARKETING, CLINICAL TRIAL, OR SURVEILLANCE STUDIES ................................................................................19
C. FOREIGN REPORTS ...........................................................................................................................................................20
D. DEATH REPORTS..............................................................................................................................................................20
E. OVERDOSE REPORTS........................................................................................................................................................20
F. LACK OF EFFECT REPORTS .............................................................................................................................................21
G. INFORMATION ON THE INTERNET....................................................................................................................................21
H. PEDIATRIC PATIENTS.......................................................................................................................................................21
I. PRESCRIPTION DRUGS MARKETED FOR HUMAN USE WITHOUT AN APPROVED APPLICATION................................22
J. ANOTHER APPLICANT=S PRODUCT.................................................................................................................................22
K. MULTIPLE SUSPECT PRODUCTS .....................................................................................................................................22
L. SUSPECT DRUGS WITH MULTIPLE NDAS OR ANDAS BY THE SAME APPLICANT ...................................................23
M. TWO OR MORE MARKETERS OF A PRODUCT ................................................................................................................23
N. UNAPPROVED INDICATIONS .............................................................................................................................................23
O. PRODUCT INTERACTIONS ................................................................................................................................................23
P. REPORTS FROM THE FDA...............................................................................................................................................23
Q. PRODUCT DEFECTS..........................................................................................................................................................24
R. REPORTING AMBIGUITIES................................................................................................................................................24
VII. CODING OF ADVERSE EXPERIENCES IN INDIVIDUAL CASE SAFETY REPORTS..................................24
VIII. REPORTING FORMATS.............................................................................................................................................25
A. FDA FORM 3500A...........................................................................................................................................................25
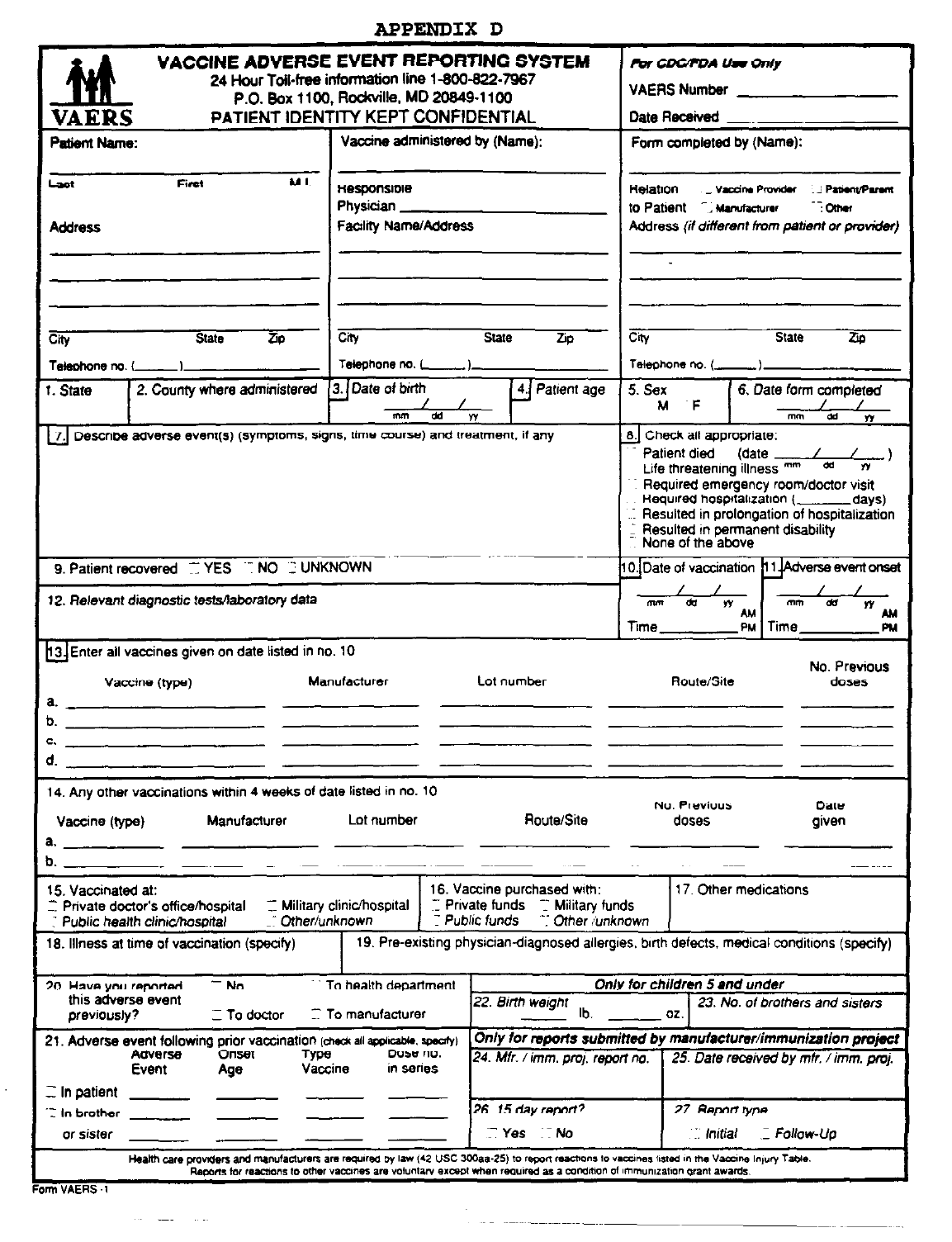
B. VAERS FORM FOR VACCINES.........................................................................................................................................28
C. CIOMS I FORM FOR FOREIGN ADVERSE EXPERIENCES................................................................................................29
D. DISTRIBUTION REPORTS FOR BIOLOGICAL PRODUCTS INCLUDING VACCINES ............................................................29
G:\4177DFT.DOC
E. ELECTRONIC SUBMISSIONS..............................................................................................................................................30
IX. HOW AND WHERE TO SUBMIT POSTMARKETING SAFETY REPORTS.........................................................30
A. H
UMAN DRUG PRODUCTS ...............................................................................................................................................30
B. H
UMAN BIOLOGICAL PRODUCTS AND VACCINES ...........................................................................................................31
X. WRITTEN PROCEDURES FOR POSTMARKETING SAFETY REPORTING.......................................................31
XI. REQUESTS FOR WAIVERS TO POSTMARKETING SAFETY REPORTING REQUIREMENTS ....................32
A. S
UBMISSION OF FDA FORM 3500A FOR NONSERIOUS, EXPECTED ADVERSE EXPERIENCES.....................................32
B. S
UBMISSION OF PSUR FORMAT FOR THE PERIODIC REPORT .......................................................................................32
C. S
UBMISSION DATE AND FREQUENCY FOR PSUR REPORTS ..........................................................................................33
D. H
OW AND WHERE TO SUBMIT WAIVER REQUESTS......................................................................................................34
XII. VALIDATION OF ADVERSE EXPERIENCE COMPUTER SYSTEMS ..............................................................34
APPENDIX A: GLOSSARY.......................................................................................................................................................35
APPENDIX B: REPORT CHECKLIST.....................................................................................................................................38
APPENDIX C: FDA FORM 500A…………………...………………………………………..………………........…...41
APPENDIX D: FORM VAERS-1……………………………………………..…………………………...….......……43
APPENDIX E: CIOMS 1 FORM…………..……………………………….……………………..…….…...........……45
APPENDIX F: ONE-PAGE FDA FORM 3500A………….…..…………………………..…….………….........…….46
Draft — Not for Implementation
G:\4177dft.doc
1
Guidance for Industry
1
1
2
Postmarketing Safety Reporting for Human Drug and3
Biological Products Including Vaccines4
5
6
7
8
9
10
11
12
13
14
15
16
I. INTRODUCTION17
18
This guidance is intended to assist applicants and other responsible parties in fulfilling the19
FDA=s existing postmarketing safety reporting requirements for human marketed drug and20
biological products at 21 CFR 310.305, 314.80, 314.98, 600.80, and 600.81.
2
Under21
these regulations, postmarketing safety reports must be submitted to the Agency for the22
following:23
24
1. Serious and unexpected adverse experiences from all sources (domestic and25
foreign)26
27
2. Spontaneously reported adverse experiences that occur domestically and that are:28
29
• Serious and expected30
• Nonserious and unexpected31
• Nonserious and expected 32
33
A. What Does This Guidance Discuss?34
35
This guidance discusses the following postmarketing reports:36
1
This guidance has been prepared by FDA=s Safety Reporting Regulations Working Group, which includes
representatives from the Center for Drug Evaluation and Research (CDER) and the Center for Biologics Evaluation
and Research (CBER).
2
The FDA is planning to propose revisions to these regulations (see section II.C in this guidance). As these
proposals are finalized the Agency will revise this guidance to provide industry with assistance in fulfilling the
new regulatory requirements.
This draft guidance, when finalized, represents the Food and Drug Administration's (FDA’s)
current thinking on this topic. It does not create or confer any rights for or on any person and
does not operate to bind FDA or the public. An alternative approach may be used if such
approach satisfies the requirements of the applicable statutes and regulations.

Draft — Not for Implementation
G:\4177dft.doc
2
37
• 15-Day Reports of Serious, Unexpected Adverse Experiences38
• Periodic Reports39
• Followup Reports40
• Distribution Reports for Biological Products Including Vaccines41
42
This guidance addresses the following regulations for the following products.
3
43
44
Regulation Product
21 CFR 310.305
Prescription drugs marketed for human use without an
an approved application
21 CFR 314.80 Human drugs with approved NDAs
21 CFR 314.98 Human drugs with approved ANDAs
21 CFR 600.80 Human biological products with approved BLAs
21 CFR 600.81 Human biological products with approved BLAs
45
If you believe the procedures described in this guidance are inapplicable to a particular46
product or that other procedures are appropriate, you should discuss the matter with the47
Agency to ensure that your procedures comply with applicable statutes and regulations.48
49
B. What Does This Guidance Not Discuss?50
51
This guidance does not discuss the following: 52
53
• IND Safety Reports (21 CFR 312.32)
4
54
• Safety Update Reports for Drugs (21 CFR 314.50(d)(5)(vi)(b))55
• Approved NDA Annual Reports (21 CFR 314.81(b)(2))56
• Approved BLA Annual Reports (21 CFR 601.28)57
58
This guidance does not apply to the following products:59
60
• In vitro diagnostic products 61
• Whole blood or its components62
• Product manufacturing defects (unless the defect is associated with an63
adverse experience in humans) 64
65
C. Good Guidance Practices66
67
The Agency's good guidance practices (GGPs) regulation
5
does not allow the use of68
mandatory language in guidances unless it is used to describe regulatory requirements. In69
3
NDA means new drug application, ANDA means abbreviated new drug application, and BLA means
biologics license application
4
IND means investigational new drug application

Draft — Not for Implementation
G:\4177dft.doc
3
most guidances, we provide the related cite whenever mandatory language is used to70
indicate the basis for the use of such language. This guidance discusses regulatory71
requirements in great detail. To avoid including the same regulatory cites repeatedly and72
to make the guidance user friendly, we will indicate at the beginning of those sections that73
include extensive discussions of regulatory requirements which cites are particularly74
relevant. The use of mandatory language (e.g., must, have to, required) will signify a75
regulatory requirement while the use of words such as should and recommend will indicate76
Agency policy.77
78
79
II. BACKGROUND80
81
The FDA has undertaken a major effort to clarify and revise its regulations regarding pre-82
and postmarketing safety reporting requirements for human drug and biological products. 83
To date, the Agency has issued a number of final rules and guidances for industry on this84
topic; several proposed rules are under development.85
86
A. Final Rules87
88
• Expedited Safety Reports for Human Drug and Biological Products89
90
In the Federal Register of October 7, 1997 (62 FR 52237), the FDA published a91
final rule amending its regulations for expedited safety reporting to implement92
certain definitions, reporting periods, and formats recommended by the International93
Conference on Harmonisation of Technical Requirements for Registration of94
Pharmaceuticals for Human Use (ICH). These recommendations are discussed in95
the ICH guidance E2A Clinical Safety Data Management: Definitions and96
Standards for Expedited Reporting; March 1, 1995.97
98
• Postmarketing Expedited Increased Frequency Reports for Human Drug and99
Biological Products100
101
In the Federal Register of June 25, 1997 (62 FR 34166), the FDA published a final102
rule revoking requirements to submit postmarketing increased frequency reports to103
the Agency in an expedited manner for human drug and biological products. 104
105
B. Guidances106
107
With regard to postmarketing safety reporting for human drug and biological products, the108
FDA has made three final guidances available:109
110
• Postmarketing Reporting of Adverse Drug Experiences (March 1992)111
5
The Agency's regulation on good guidance practices published on September 19, 2000 (21 CFR 10.115; 65
FR 56468).

Draft — Not for Implementation
G:\4177dft.doc
4
112
• Guideline for Adverse Experience Reporting for Licensed Biological113
Products (October 1993)114
115
• Postmarketing Adverse Experience Reporting for Human Drug and116
Licensed Biological Products: Clarification of What to Report (August 27,117
1997). 118
119
When finalized, this guidance will replace the three guidances listed above and will reflect120
the new regulatory requirements in the final rules of June 25, 1997, and October 7, 1997.121
122
C. Proposed Rules123
124
The Agency currently is in the process of developing proposed rules to further amend its125
safety reporting requirements for human drug and biological products. Many of the126
provisions in these proposed rules will be based on recommendations developed by ICH. 127
For instance, the Agency is planning to propose additional amendments to its expedited128
safety reporting regulations based on the ICH E2A guidance.129
130
In addition, the FDA is planning, as indicated in the final rule of October 7, 1997, to131
repropose amendments to its postmarketing periodic safety reporting requirements that132
were initially proposed in the Federal Register of October 27, 1994 (59 FR 54046). The133
new postmarketing periodic safety reporting proposals will be based on recommendations134
in the ICH guidance E2C Clinical Safety Data Management: Periodic Safety Update135
Reports for Marketed Drugs (May 19, 1997). 136
137
The Agency also is planning to issue a proposal requiring the electronic submission of138
postmarketing safety reports consistent with recommendations developed by ICH.
6
139
140
As these proposed rules are finalized, this postmarketing safety reporting guidance for141
human drug and biological products will be revised to provide industry with assistance in142
fulfilling the new regulatory requirements.143
144
145
III. WHO MUST REPORT146
147
According to the regulations, the following persons have postmarketing safety reporting148
responsibilities:149
150
• Manufacturers are required to submit postmarketing expedited safety reports to the151
FDA for prescription drug products marketed for human use without an approved152
application (§ 310.305).153
6
See advance notice of proposed rulemaking on electronic reporting of postmarketing adverse drug
reactions; request for comments, 63 FR 59746, November 5, 1998.

Draft — Not for Implementation
G:\4177dft.doc
5
154
• Applicants (individual or corporate entity that holds an NDA or ANDA) are required155
to submit postmarketing safety reports to the FDA for human drug products with156
approved NDAs (§ 314.80) and ANDAs (§ 314.98).157
158
• Licensed manufacturers (individual or corporate entity that holds a BLA) are159
required to submit postmarketing safety reports to the FDA for human licensed160
biological products with approved BLAs (§§ 600.80 and 600.81).161
162
• Any person whose name appears on the label of a marketed drug as its packer or163
distributor (§ 310.305(c)(1)(i)) or manufacturer, packer, or distributor164
(§ 314.80(c)(1)(iii)) has postmarketing safety reporting responsibilities.165
166
• Any person whose name appears on the label of a licensed biological product as its167
manufacturer, packer, distributor, shared manufacturer, joint manufacturer, or any168
other participant involved in divided manufacturing has postmarketing safety169
reporting responsibilities (§ 600.80(c)(1)(iii)).170
171
For the purposes of this guidance, the term applicant includes all persons with172
postmarketing safety reporting responsibilities under §§ 310.305, 314.80, 314.98, 600.80,173
and 600.81.174
175
According to the regulations at §§ 310.305(d), 314.80(f), and 600.80(f), if an applicant176
becomes aware of a reportable adverse experience, the applicant is responsible for177
preparing a postmarketing safety report and submitting it to the FDA. Applicants should178
not assume that their responsibilities are fulfilled if they ask the person who pointed out a179
reportable adverse experience to submit a safety report to the FDA.180
181
182
IV. WHAT DO I REPORT?183
184
The following paragraphs discuss the types of adverse experiences that must be reported185
to the FDA under §§ 310.305, 314.80, 314.98, and 600.80. This section also describes186
the minimum data elements that should be included in an individual case safety report.187
188
An adverse experience is any undesirable event that is associated with the use of a drug189
or biological product in humans whether or not considered product-related by the190
applicant.
7
An individual case safety report describes an adverse experience(s) for a191
patient or subject. Individual case safety reports of domestic adverse experiences for192
marketed human drug and biological products, except vaccines, must be submitted to the193
FDA on FDA Form 3500A; a Vaccine Adverse Event Reporting System (VAERS) form194
must be used for adverse experiences associated with the use of vaccines. Individual195
case safety reports of foreign adverse experiences can be submitted on FDA Form 3500A196
7
See Appendix A for definition of adverse experience. (See also '' 310.305(b), 314.80(a) and 600.80(a).)

Draft — Not for Implementation
G:\4177dft.doc
6
(VAERS form for vaccines) or, if preferred, on a Council for International Organizations for197
Medical Sciences (CIOMS) I form. See section VIII in this guidance for discussion of198
reporting formats for individual case safety reports. 199
200
A. Type of Adverse Experiences201
202
1. Adverse Experiences that are Serious and Unexpected from All Sources203
(Domestic and Foreign)
8
204
205
Serious and unexpected adverse experiences from all sources, whether domestic206
or foreign, must be submitted to the FDA. Possible sources include, for example,207
scientific literature, postmarketing studies, or commercial marketing experience. 208
209
Scientific literature reports include published and unpublished scientific papers that210
are known to the applicant (see section VI.A in this guidance for reporting of211
adverse experiences from the scientific literature).212
213
Postmarketing studies include in vitro, animal, clinical, and epidemiological or214
surveillance investigations (see section VI.B in this guidance for reporting of215
adverse experiences from studies). Adverse experiences from studies must only216
be submitted to the FDA if the applicant believes that there is a reasonable217
possibility that the drug or biological product caused the adverse experience (see218
§§ 310.305(c)(1)(ii), 314.80(e)(1) and 600.80(e)(1)).219
220
2. Other Spontaneously Reported Adverse Experiences (Domestic Only)
9
221
222
Adverse experiences occurring in the United States from commercial marketing223
experience must be submitted to the FDA if they are spontaneously reported to224
applicants and are:225
226
• serious and expected227
• nonserious and unexpected, or228
• nonserious and expected229
230
Applicants can request a waiver of the requirement to submit individual case safety231
reports of nonserious, expected adverse experiences for drugs and certain232
biological products (see section XI.A in this guidance on waiver requests).233
234
3. Serious Adverse Experiences
10
235
8
The requirements for reports of serious, unexpected adverse experiences can be found in §§ 310.305(c),
314.80(c)(1) and 600.80(c)(1).
9
The requirements for reports describing these adverse experiences can be found in §§ 314.80(c)(2) and
600.80(c)(2).

Draft — Not for Implementation
G:\4177dft.doc
7
236
The outcome of an adverse experience must be determined before a report can be237
identified as serious. A serious report must have one or more of the following238
outcomes:239
240
• Death241
• Life-threatening adverse experience242
• Initial inpatient hospitalization or prolongation of hospitalization243
• Significant or persistent disability/incapacity244
• Congenital anomaly/birth defect (including that occurring in a fetus)245
• Important medical event based upon appropriate medical judgment that may246
jeopardize the patient or subject and may require medical or surgical247
intervention to prevent one of the other outcomes listed in the definition of248
serious. 249
250
A patient admitted to a hospital for 1 or more days as a result of an adverse251
experience, even if released on the same day, would qualify for the initial inpatient252
hospitalization outcome. An emergency room visit that results in admission to the253
hospital would also qualify for the initial inpatient hospitalization outcome. 254
However, emergency room visits that do not result in admission to the hospital255
would not qualify for this outcome and, instead, should be evaluated for one of the256
other outcomes in the definition of serious (e.g., life-threatening adverse257
experience, important medical event).258
259
Persons incarcerated because of actions allegedly caused by a drug (e.g.,260
psychotropic drugs and rage reactions) have sustained a substantial disruption in261
their ability to conduct normal life functions. Thus, these adverse experiences would262
qualify for the significant or persistent disability/incapacity outcome.263
264
Important medical events would include allergic bronchospasm requiring intensive265
treatment in an emergency room or at home, blood dyscrasias or convulsions that266
do not result in inpatient hospitalization, or the development of drug dependency or267
drug abuse. Applicants should mark the "other" box in item B2 of FDA Form 3500A268
for adverse experiences identified as important medical events.269
270
Applicants should actively seek the outcome for a suspected serious adverse271
experience reported to them. If unable to initially determine the outcome for an272
adverse experience, an applicant should continue to actively seek information in an273
attempt to determine an outcome. For a serious adverse experience that was not274
initially reported to the applicant by a health care professional (e.g., report from a275
consumer), the applicant should actively pursue contacting the health care276
10
See Appendix A for definition of serious adverse experience. (See also §§ 310.305(b), 314.80(a) and
600.80(a).)

Draft — Not for Implementation
G:\4177dft.doc
8
professional associated with the care of the patient to gather further medical277
perspective on the case.278
279
4. Unexpected and Expected Adverse Experiences
11
280
281
The current FDA-approved labeling for the human drug or biological product should282
be used as the reference document to determine whether an adverse experience is283
unexpected or expected.
An adverse experience would be considered unexpected284
if it is not included in the product’s current FDA-approved labeling and expected if it285
is included in this document.286
287
5. Spontaneous Report
12
288
289
Spontaneous reports are unsolicited communications from individuals (e.g., health290
care professional, consumer) to applicants that concern adverse experiences. 291
Spontaneous reports should not include adverse experiences identified from292
information solicited by applicants such as individual cases or findings derived from293
a study (e.g., any organized data collection scheme).294
295
B. Data Elements to Include in a Postmarketing Individual Case Safety Report296
297
Before considering any clinical incident for submission to the FDA in an individual case298
safety report, applicants should, at a minimum, have knowledge of the following four data299
elements:300
301
1. An identifiable patient302
2. An identifiable reporter303
3. A suspect drug or biological product304
4. An adverse experience or fatal outcome suspected to be due to the suspect305
drug or biological product306
307
If any one of these basic elements remains unknown after being actively sought by the308
applicant, a report on the incident should not be submitted to the FDA because reports309
without such information make interpretation of their significance difficult, at best, and310
impossible, in most instances. Instead, the applicant should maintain records of its efforts311
to obtain the basic elements for an individual case in its corporate drug or biological312
product safety files. If an applicant submits a report to the FDA that lacks any of the four313
basic elements, it will be returned to the applicant marked insufficient data for a report. 314
315
11
See Appendix A for definitions of unexpected and expected adverse experiences. (See also §§
310.305(b), 314.80(a) and 600.80(a).)
12
See Appendix A for definition of spontaneous report.

G:\4177dft.doc
9
An applicant that is actively seeking information on an adverse experience should use316
direct verbal contact with the initial reporter of the adverse experience (e.g., in person, by317
telephone or other interactive means such as a videoconference). The applicant should not318
merely send the initial reporter a letter requesting information concerning the adverse319
experience. Applicants should use a health care professional (e.g., physician, physician320
assistant, dentist, pharmacist, nurse) for contacts with initial reporters because such321
persons should be able to understand the medical consequences of the case and ask322
appropriate questions to acquire relevant information rapidly to determine the significance323
of the case.324
325
With regard to an identifiable patient, reports of the type Asome patients got anaphylaxis@326
should be excluded until further information about the patients is obtained. A report stating327
that Aan elderly woman had anaphylaxis@ or a Ayoung man experienced anaphylaxis@ should328
be included because there is enough information to suspect that specific patients were329
involved. Patients should not be identified by name or address. Instead, the applicant330
should assign a unique code (e.g., patient initials) to each report. 331
For spontaneous reports, the applicant should assume that an adverse experience or fatal332
outcome was suspected to be due to the suspect drug or biological product (implied333
causality). For clinical studies, an adverse experience or fatal outcome need not be334
submitted to the FDA unless the applicant concludes that there is a reasonable possibility335
that the product caused the adverse experience or fatal outcome (see §§ 310.305(c)(1)(ii),336
314.80(e)(1) and 600.80(e)(1)). An adverse experience should, at a minimum, consist of337
signs (including abnormal laboratory findings, if appropriate), symptoms, or disease338
diagnosis (including any colloquial descriptions obtained) for purposes of reporting. Thus,339
a report stating that a patient Aexperienced unspecified injury,@ or a patient Asuffered340
irreparable damages@ should not be included until more specific information about the341
adverse experience can be determined.342
343
344
V. TYPE OF REPORTS345
346
The following paragraphs discuss the types of postmarketing safety reports that must be347
submitted to the FDA based on the regulations as listed.348
349
A. 15-Day Reports of Serious, Unexpected Adverse Experiences
13
350
351
Individual case safety reports of serious, unexpected adverse experiences from all sources352
(domestic and foreign) must be reported to the FDA as soon as possible, but in no case353
later than 15 calendar days of initial receipt of the information by the applicant. See section354
VIII in this guidance for discussion of reporting formats for individual case safety reports.355
356
13
The requirements for 15-Day Reports can be found in §§ 310.305(a), (c)(1)(i) and (d)(1), 314.80(c)(1)(i) and
(f)(1), and 600.80(c)(1)(i) and (f)(1).
Draft — Not for Implementation
G:\4177dft.doc
10
An applicant should not wait for the initial reporter of a serious, unexpected adverse357
experience to send them written information about the experience before submitting a 15-358
day report to the FDA. An applicant can and should submit a 15-day report to the FDA359
based only on verbal information.360
361
1. Determination of 15-Day Reporting Period362
363
Serious, unexpected adverse experiences must be submitted to the FDA no later364
than 15 calendar days of initial receipt of the information by the applicant. For365
reporting purposes, this information should include, at a minimum, the four basic366
elements (i.e., an identifiable patient, an identifiable reporter, a suspect drug or367
biological product, and a serious, unexpected adverse experience). The date the368
company has knowledge of these four basic elements should be entered into item369
G4 of FDA Form 3500A or Box 25 of the VAERS form (i.e., this date represents370
Day 0 of the 15-day time clock).371
372
If the 15th calendar day occurs on a weekend or U.S. Federal holiday, the 15-day373
report should be submitted the first working day after the weekend or U.S. Federal374
holiday.375
376
The applicant should exercise due diligence to acquire all the information for an377
individual case safety report immediately upon receipt of a suspected serious,378
unexpected adverse experience (e.g., completion of all the applicable elements on379
FDA Form 3500A). The applicant should maintain records of its efforts to obtain380
this information and should include in the narrative section of FDA Form 3500A (i.e.,381
item B5), a chronological description of these efforts if there is a delay in obtaining382
such information.383
384
When an applicant receives a report of a serious, unexpected adverse experience385
but it is not possible to complete all the applicable elements for an individual case386
safety report within 15 calendar days, a preliminary report that contains at least the387
four basic elements should be submitted. Additional followup information should be388
actively sought and submitted within 15 calendar days after obtaining the new389
information (see section V.C in this guidance for discussion of followup reports).390
391
For foreign reports, the 15-day time clock begins when the applicant or its foreign392
affiliate has received the four basic elements for a 15-day report. Applicants should393
therefore establish effective mechanisms to ensure rapid information transfer from394
their foreign affiliates.395
396
2. Supporting Documentation397
398
For individual case safety reports of serious, unexpected adverse experiences, the399
FDA encourages applicants to include relevant hospital discharge summaries and400
autopsy reports/death certificates. Applicants should also include in their report a401

Draft — Not for Implementation
G:\4177dft.doc
11
list of other relevant documents (e.g., medical records, relevant laboratory data,402
electrocardiograms, and other concise critical clinical data) maintained in their403
corporate drug or biological product safety files. The FDA can request that copies404
of one or more of these documents be provided to the Agency. Applicants should405
submit copies of these documents to the Agency within 5 calendar days after406
receipt of the request.407
408
3. Report Identification409
410
Fifteen-day reports must be submitted in duplicate under separate cover411
prominently identified as "15-Day Alert Report." For this purpose, the “15-Day Alert412
Report” identification should be included on the outside envelope. 413
414
For prescription drugs marketed for human use without an approved application, a415
single copy of the 15-day report and a copy of the U.S. labeling must be submitted.416
These reports should be marked on the outside envelope with "15-Day Alert Report417
- 310.305."418
419
Multiple 15-day reports and 15-day followup reports can be submitted in the same420
envelope, but they should not be stapled together (see section V.C for discussion of421
followup reports).422
423
B. Periodic Reports
14
424
425
The following paragraphs discuss the reporting frequency for submission of periodic426
reports and the content of these reports. See section XI in this guidance for requests for427
waivers of the requirement to submit postmarketing periodic safety reports (e.g., waiver to428
use periodic safety update report (PSUR) format recommended by ICH for periodic report429
instead of format described in the regulations, waiver to submit individual cases of430
nonserious, expected adverse experiences in periodic report).431
432
1. Timing of Postmarketing Periodic Reports433
434
Postmarketing periodic reports are required to be submitted to the FDA for each435
approved NDA, ANDA, and BLA and are due quarterly for the first 3 years after U.S.436
approval of the application and annually thereafter. If marketing is delayed, these437
reports should still be submitted quarterly for the first 3 years of marketing. Upon438
written notice, the FDA may extend or reestablish the requirement that an applicant439
submit quarterly reports or require that the applicant submit periodic reports at440
different time intervals.441
442
Periodic reports due quarterly must be submitted within 30 calendar days of the last443
day of the reporting quarter. Reports due annually must be submitted each year444
14
The requirements for periodic reports can be found in '' 314.80(c)(2) and 600.80(c)(2).

Draft — Not for Implementation
G:\4177dft.doc
12
within 60 calendar days of the anniversary date of U.S. approval of the application445
for the drug or biological product (i.e., NDA, ANDA, BLA).446
447
Periodic submissions should be clearly marked "Periodic Adverse Experience448
Submission" on the front cover of each volume. Each page of the periodic report449
should be numbered and include the name and NDA or ANDA number if the450
periodic report is for a drug product; the name and submission tracking number451
(STN) should be used if the periodic report is for a biological product (a STN for a452
biological product can be found on the Internet at www.fda.gov/cber/stn/stn.htm).453
454
2. Content of a Postmarketing Periodic Report455
456
The regulations require a postmarketing periodic report to contain:457
458
• a narrative summary and analysis of the information in the report and an analysis459
of the 15-day Alert reports submitted during the reporting interval460
• an FDA Form 3500A for each spontaneously reported adverse experience461
occurring in the United States that was not reported in a 15-day Alert report462
• a history of actions taken since the last report because of adverse experiences. 463
464
The information contained within a postmarketing periodic report should be divided465
into four sections in the order described below and should be clearly separated by466
an identifying tab. If information for one of these sections is not included, the467
applicant should simply explain why the information is not provided.468
469
a. Section 1: Narrative summary and analysis470
471
A narrative summary and analysis of the information in the postmarketing472
periodic report and an analysis of the 15-day reports (i.e., serious,473
unexpected adverse experiences) submitted during the reporting period474
must be provided and should include:475
476
• The number of non-15-day
15
initial adverse experience reports and477
the number of non-15-day followup reports contained in this periodic478
report and the time period covered by the periodic report.479
480
• A line listing of the 15-day reports submitted during the reporting481
period. This line listing should include the manufacturer report482
number, adverse experience term(s), and the date the 15-day report483
was sent to the FDA.484
485
15
These include serious and expected adverse experiences, nonserious and unexpected adverse
experiences, and nonserious and expected adverse experiences.

G:\4177dft.doc
13
• A summary tabulation by body system (e.g., cardiovascular, central486
nervous system, endocrine, renal) of all adverse experience terms487
and counts of occurrences submitted during the reporting period. The488
information should be taken from :489
490
- 15-day reports submitted to the FDA;491
- non-15-day reports submitted in the periodic report;492
- reports forwarded to the applicant by the FDA; and493
- any nonserious, expected adverse experiences not submitted to494
the FDA but maintained on file by the applicant.495
496
For the adverse experience term product interaction, the interacting497
products should be identified in the tabulation.498
499
• A summary listing of the adverse experience reports in which the drug500
or biological product was listed as one of the suspect products, but501
the report was filed to another NDA, ANDA, or BLA held by the502
applicant.503
504
• A narrative discussion of the clinical significance of the 15-day reports505
submitted during the reporting period and of any increased reporting506
frequency of serious, expected adverse experiences when, in the507
judgment of the applicant, it is believed the data reflect a clinically508
meaningful change in adverse experience occurrence. This narrative509
should assess clinical significance by type of adverse experience,510
body system, and overall product safety relating the new information511
received during this reporting period to what was already known512
about the product. The narrative should also state what further513
actions, if any, the applicant plans to undertake based on the514
information gained during the reporting period and include the time515
period for completing the actions (i.e., when the applicant plans to516
start and finish the action and submit the information to the Agency). 517
518
• The narrative discussion should indicate, based on the information519
learned during the reporting period, whether the applicant believes520
either that (1) no change in the product’s current approved labeling is521
warranted or (2) there are safety-related issues that need to be522
addressed in the approved product labeling. If changes in the523
approved product labeling are under consideration by the FDA, the524
applicant should state in the narrative the date and number of the525
supplemental application submitted to address the labeling changes.526
527
Draft — Not for Implementation
G:\4177dft.doc
14
b. Section 2: Narrative discussion of actions taken528
529
A narrative discussion of actions taken must be provided, including any530
labeling changes and studies initiated since the last periodic report. This531
section should include:532
533
• A copy of current U.S. product labeling534
535
• A list of any labeling changes made during the reporting period536
537
• A list of studies initiated538
539
• A summary of important foreign regulatory actions (e.g., new540
warnings, limitations in the indications and use of the product)541
542
• Any communication of new safety information (e.g., a Dear Doctor543
letter)544
545
c. Section 3: Index line listing546
547
An index line listing of FDA Form 3500As or VAERS forms included in548
section 4 of the periodic report must be provided. The line listing for each549
FDA Form 3500A or VAERS form submitted should include:550
551
• Manufacturer report number552
553
• Adverse experience term(s)554
555
• Page number of FDA Form 3500A or VAERS form as located in the556
periodic report557
558
• Identification of interacting products for any product interaction listed559
as an adverse experience.560
561
d. Section 4: FDA Form 3500As or VAERS forms562
563
FDA Form 3500As or VAERS forms must be provided for the following564
spontaneously reported adverse experiences that occurred in the United565
States during the reporting period:566
567
• Serious and expected568
569
• Nonserious and unexpected570
571

Draft — Not for Implementation
G:\4177dft.doc
15
• Nonserious and expected572
573
Applicants are encouraged to request a waiver of the requirement to submit574
individual case safety reports of nonserious, expected adverse experiences575
for drugs and certain biological products as described below (see section576
XI.A in this guidance). 577
578
Adverse experiences due to a failure to produce the expected579
pharmacologic action (i.e., lack of effect) should be included in this section580
(see section VI.F in this guidance). 581
582
For individual case safety reports of serious, expected adverse experiences,583
the FDA encourages applicants to include relevant hospital discharge584
summaries and autopsy reports/death certificates, as well as lists of other585
relevant documents as described for 15-day reports of serious, unexpected586
adverse experiences (see section V.A.2 in this guidance).587
588
Initial non-15 day reports should be included in the periodic report in a589
separate section from non-15 day followup reports (see the following section590
V.C for discussion of non-15 day followup reports). All initial and followup591
information obtained for an adverse experience with a given periodic592
reporting period should be combined and submitted in the periodic report as593
one initial non-15 day report (i.e., an initial non-15 day report and a non-15594
day followup report describing the same adverse experience should not be595
submitted in the same periodic report). 596
597
An FDA Form 3500A or VAERS form for a serious, unexpected adverse598
experience should not be included in a periodic report because this adverse599
experience should have been previously submitted to the FDA as a 15-day600
report.601
602
If no adverse experiences were identified for the human drug or biological product603
for the time period involved and no regulatory actions concerning safety were taken604
anywhere in the world where the product is marketed, the periodic report should605
simply state this and be submitted to the FDA along with a copy of the current U.S.606
labeling.607
608
C. Followup Reports
16
609
610
The following paragraphs discuss the content of and reporting considerations for 15-day611
followup reports that are submitted in an expedited manner and non-15 day followup612
reports that are submitted as part of a postmarketing periodic report. A followup report613
provides information about an adverse experience that has been previously reported as an614
16
The requirements for followup reports can be found in '' 310.305(c)(2), 314.80(c) and 600.80(c).
Draft — Not for Implementation
G:\4177dft.doc
16
initial report with a unique manufacturer report number. The followup report should be615
identified with the same unique manufacturer report number as the initial report.616
617
A 15-day followup report must be submitted within 15 calendar days of receipt of new618
information on a 15-day report. Followup information to adverse experiences submitted619
initially in a periodic report can be submitted in the next periodic report.620
621
1. Content of Followup Reports622
623
A followup report should provide a complete picture of the current understanding of624
the adverse experience. Relevant information from the initial report should be625
combined with the followup information to present an accurate and comprehensive626
description of the adverse experience as it is understood at the time of the followup.627
Information from the initial report later found to be inaccurate should not be repeated628
in the followup report. All new information including correction of previously629
submitted inaccurate information that is included in a followup report should be630
highlighted (e.g., with an asterisk, underlined).631
632
The narrative section of the followup report should be concise (i.e., item B5 of FDA633
Form 3500A) because the FDA’s adverse event reporting database (AERS) is634
limited for this section of the form. 635
636
For serious adverse experiences, applicants should exercise due diligence in637
obtaining followup information for the purposes of completing all the applicable638
elements for an individual case safety report (e.g., FDA Form 3500A). For adverse639
experiences that are determined to be nonserious and for which the four basic640
elements are known (see section IV.B), additional followup is not necessary.641
642
Any attachments submitted with an initial report (e.g. scientific journal articles,643
hospital discharge summaries) should not be resubmitted with a followup report.644
645
2. Reporting Considerations646
647
A copy of the initial report or a previous followup report should not be sent with the648
latest followup report. Fifteen-day followup reports should not be submitted in the649
same envelope with periodic reports.650
651
If the initial report was submitted as a 15-day report, the followup report should be652
submitted as a 15-day followup report even if the followup information shows that the653
adverse experience was expected or not serious. All subsequent followup reports654
for adverse experiences that are expected or not serious should be submitted in655
periodic reports. A 15-day followup report should be submitted if the adverse656
experience is found to be serious and unexpected, even if the original report was657
not submitted as a 15-day report.658
659
Draft — Not for Implementation
G:\4177dft.doc
17
If a new adverse experience occurs that is associated with the initial adverse660
experience, a followup report should be submitted. However, if the new adverse661
experience is not associated with the initial adverse experience (e.g., occurs after a662
subsequent administration of the product), an initial report with a new manufacturer663
report number should be submitted for the new adverse experience. In these cases,664
the applicant should consider the clinical relevance of the adverse experiences to665
each other when determining whether an initial report or followup report should be666
submitted.667
668
Followup reports should not be submitted if additional relevant information is not669
obtained for the adverse experience. However, as described in the regulations,670
applicants should maintain records of their efforts to obtain additional information,671
particularly for serious adverse experiences. FDA may request this documentation.672
673
3. Reporting Forms674
675
For followup reports, particular attention should be paid to completing the following676
items on FDA Form 3500A:677
678
• Item G3 - Mark health professional if at any time a health professional679
provided information for the report.680
• Item G4 - Use the date the followup information was received by the681
applicant.682
• Item G7- Mark followup, and indicate whether this is the 1st, 2nd, 3rd,683
... followup report.684
• Item G9 - Use the same unique manufacturer report number assigned685
to the initial report. This is essential to prevent duplicate counting of686
reports and to ensure that the followup information is coupled with the687
correct initial report.688
689
For followup reports, particular attention should be paid to completing the following690
items on the VAERS form for vaccines:691
692
• Top right - Indicate the name of the person who provided information693
for the report.694
• Box 24 - Use the same manufacturer report number assigned to the695
initial report. This is essential to prevent duplicate counting of reports696
and to ensure that the followup information is coupled with the correct697
initial report.698
• Box 25 - Use the date the followup information was received by the699
applicant.700
• Box 27 - Mark followup, and indicate whether this is the 1st, 2nd, 3rd,701
... followup report.702
703

Draft — Not for Implementation
G:\4177dft.doc
18
4. Report Identification704
705
Fifteen-day followup reports must be submitted in duplicate under separate cover706
prominently identified as "15-Day Alert Report-Followup." For this purpose, the “15-707
Day Alert Report-Followup” identification should be included on the outside708
envelope.709
710
D. Distribution Reports for Biological Products Including Vaccines711
712
This section is based primarily on regulations in § 600.81. These regulations only apply to713
human biological products with approved BLAs. Unless otherwise notified by the Director,714
Center for Biologics Evaluation and Research, an applicant must submit at periodic715
intervals two copies of a report containing information about the quantity of the product716
distributed domestically (including distributors) under the BLA.717
718
Distribution reports are due within the first 6 months after approval of a BLA, and,719
subsequently, at 6-month intervals. Upon written notice, the FDA can require that the720
applicant submit reports under this section at alternate times.721
722
The report must include the bulk lot, fill lot, and label lot numbers for the total number of723
dosage units of each strength or potency distributed (e.g., 50,000 per 10-milliliter vials),724
labeled date of expiration, and date of distribution of fill lot or label lot. The report must also725
include information about any significant amount of a fill lot or label lot that may have been726
returned. Disclosure of financial or pricing data is not required. According to the727
regulations, the FDA can require submission of more detailed product distribution728
information, if needed.729
730
See section VIII.E in this guidance for a suggested reporting format for distribution reports.731
732
733
VI. SPECIAL REPORTING SITUATIONS734
735
A. Scientific Literature Reports
17
736
737
Serious, unexpected adverse experiences reported in the scientific literature (or in an738
unpublished scientific paper) that are known to the applicant must be submitted as 15-day739
reports on an FDA Form 3500A or comparable format. Applicants can use literature740
search services (e.g., Weekly Reactions) to identify adverse experiences in the scientific741
literature. A copy of the article or manuscript must be attached to the completed FDA742
Form 3500A; it is not sufficient to submit only abstracts of articles. All reports from the743
scientific literature and unpublished scientific papers should be marked Literature in item744
G3 of FDA Form 3500A.745
17
The requirements for scientific literature reports can be found in '' 314.80(c)(1)(i), 314.80(d),
600.80(c)(1)(i), and 600.80(d).

Draft — Not for Implementation
G:\4177dft.doc
19
746
A separate FDA Form 3500A should be completed for each identifiable patient that747
experiences a serious, unexpected adverse experience. Thus, if an article describes six748
patients that experience a given serious, unexpected adverse experience, six FDA Form749
3500As should be completed. In such cases, a copy of the article should be attached only750
to one of the FDA Form 3500As. All other FDA Form 3500As submitted for the article751
should reference the manufacturer report number of the FDA Form 3500A that has the752
copy of the article attached.753
754
If multiple products are mentioned in the article, an FDA Form 3500A should be submitted755
only by the applicant whose product is the suspect drug. The suspect product is that756
identified by the article=s author and is usually mentioned in the article=s title. If the757
applicant believes that the suspect product is different from the one identified by the author758
of the article, the applicant should indicate such information in the narrative section of the759
FDA Form 3500A.760
761
Reports of serious, unexpected adverse experiences described in the scientific literature762
should be submitted for products that have the same active moiety as a product marketed763
in the United States. This is true even if the excipient, dosage forms, strengths, routes of764
administration, and indications vary.765
766
When a serious, unexpected adverse experience is based on a foreign language article or767
manuscript, the applicant should translate the publication into English promptly. The original768
article or unpublished scientific paper and translation should be attached to the submitted769
FDA Form 3500A.770
771
B. Postmarketing, Clinical Trial, or Surveillance Studies
18
772
773
For the purposes of this section, a study refers to the systematic collection of data involving774
solicitation of adverse experience information (e.g., derived from a clinical trial, patient775
registry). Adverse experiences incidental to other types of studies not involving monitoring776
adverse experiences of products should be treated as spontaneous reports (see Appendix777
A in this guidance for definition of spontaneous report). For purposes of safety reporting,778
reports of suspected adverse experiences obtained from company sponsored patient779
support programs and disease management programs should be handled as if they were780
study reports and not as spontaneous reports.781
782
Serious, unexpected adverse experiences that occur during a study must be submitted as783
15-day reports. These adverse experiences are only required to be reported if there is a784
reasonable possibility that the drug or biological product caused the adverse experience.785
786
18
The requirements for reporting adverse experiences from studies can be found in '' 310.305(c)(1),
314.80(c)(2)(iii), 314.80(e)(1), 600.80(c)(2)(iii), and 600.80(e)(1).

Draft — Not for Implementation
G:\4177dft.doc
20
Adverse experiences occurring with marketed drug or biological products during IND trials787
must also be submitted, as prescribed under ' 312.32, to the FDA new drug review788
division in the Center for Drug Evaluation and Research or the product review office in the789
Center for Biologics Evaluation and Research that has responsibility for oversight of the790
IND.791
792
For each adverse experience, a suspect product should be identified. Reports from793
blinded studies should be submitted only after the code is broken. The blind should always794
be broken for each patient or subject that experiences a serious, unexpected adverse795
experience unless arrangements have been made otherwise with the responsible FDA796
review division. Exceptions to breaking the blind usually involve situations in which797
mortality or certain serious morbidities are indeed the clinical endpoint. This is consistent798
with the ICH E2A guidance.799
800
C. Foreign Reports
19
801
802
Foreign reports of serious, unexpected adverse experiences must be submitted as 15-day803
reports. Other foreign reports, including serious and expected, nonserious and unexpected,804
and nonserious and expected adverse experiences are not required to be submitted.805
806
Reports of foreign serious, unexpected adverse experiences should be submitted for807
products that have the same active moiety as a product marketed in the United States.808
This is true even if the excipient, dosage forms, strengths, routes of administration, and809
indications vary. When a foreign report is submitted on a product that is not identical to a810
product marketed in the United States, item C1 of FDA Form 3500A should contain the811
foreign trade name, the generic name, and the NDA number for the product with the same812
active moiety that is marketed in the United States.813
814
D. Death Reports815
816
Death is always a serious outcome (see definition of serious in Appendix A of this817
guidance and at '' 310.305(b), 314.80(a) and 600.80(a)). Thus, if death is associated818
with an unexpected adverse experience, or if death is associated with an expected819
adverse experience but the labeling does not specifically state that the adverse experience820
may be associated with a fatal outcome, a 15-day report should be submitted.821
822
E. Overdose Reports823
824
Reports of overdose should be submitted only when the overdose is associated with an825
adverse experience. If the adverse experience associated with the overdose is serious826
and unexpected, a l5-day report should be completed. If the adverse experience is serious827
and expected, nonserious and unexpected, or nonserious and expected, a non-15 day828
19
The requirements for reporting of foreign adverse experiences can be found in '' 310.305(c)(1)(i),
314.80(c)(2)(iii) and 600.80(c)(2)(iii).
Draft — Not for Implementation
G:\4177dft.doc
21
report should be submitted in the periodic report for spontaneously reported domestic829
cases.830
831
F. Lack of Effect Reports832
833
The definition of adverse experience includes any failure of expected pharmacological834
action that is synonymous with lack of effect (see definition of adverse experience in835
Appendix A of this guidance and at '' 310.305(b), 314.80(a) and 600.80(a)). All836
spontaneously reported cases of a lack of effect that occur in the United States should be837
reported on FDA Form 3500A and submitted in the periodic report with other adverse838
experiences. The lot number of the suspect product should be included in item C6 of FDA839
Form 3500A.840
841
If the report of lack of effect is for an unapproved indication, the event should not be842
reported to the FDA as an individual case safety report. Instead, this information should be843
included in the narrative summary section of the periodic report.844
845
G. Information on the Internet846
847
Adverse experience information that is submitted to an applicant via the Internet (e.g., e-848
mail) should be reported to the FDA if the applicant has knowledge of the four basic849
elements for an individual case safety report (see section IV.B in this guidance). 850
Applicants should review any Internet sites sponsored by them for adverse experience851
information, but are not responsible for reviewing any Internet sites that are not sponsored852
by them. However, if an applicant becomes aware of an adverse experience on an Internet853
site that it does not sponsor, the applicant should review the adverse experience and854
determine if it should be reported to the FDA.855
856
H. Pediatric Patients857
858
For children under 3 years of age, the child=s date of birth and age in days or months (e.g.,859
15 months) should be included under item A2 of FDA Form 3500A. The word days or860
months should be clearly written. For all pediatric patients, body weight (item A4 of FDA861
Form 3500A) and dose (item C2 of FDA Form 3500A) should be included.862
863
For reports of a congenital anomaly, the age and sex of the infant should be included.864
Followup reports for the infant should be considered followup to the initial report; followup865
for the mother should be submitted as a new initial individual case safety report on a866
separate FDA Form 3500A. The date that the congenital anomaly is detected should be867
used as the event onset date (e.g., birth date of the infant, date pregnancy is terminated,868
date congenital anomaly is detected by ultrasound or other diagnostic technique). This869
date should be used in item B3 of FDA Form 3500A.870
871

G:\4177dft.doc
22
I. Prescription Drugs Marketed for Human Use Without an Approved872
Application
20
873
874
For prescription drugs marketed for human use without an approved NDA or ANDA, all875
serious, unexpected adverse experiences must be reported to the FDA on an FDA Form876
3500A within 15 calendar days.
These reports must be submitted in SINGLE copy under877
separate cover. The report should be marked on the outside envelope "15-Day Alert878
Report - 310.305." A copy of the U.S. product labeling must accompany each report. 879
880
Postmarketing periodic reports should not be submitted for these drugs.881
882
J. Another Applicant==s Product883
884
Reports of adverse experiences in which the initial reporter identifies the suspect product885
as one marketed by another applicant should be promptly forwarded to that applicant. An886
applicant who receives a report of an adverse experience regarding one of its products887
from another applicant must submit the report to the FDA within the same time constraints888
applicable to any report received from a third party (see section VI.K in this guidance).889
890
An applicant should only submit a report of an adverse experience to the FDA for a891
suspect product marketed by another applicant if the applicant of the suspect product is892
unknown or the report is for a serious, unexpected adverse experience occurring during the893
conduct of a study.894
895
K. Multiple Suspect Products896
897
If a reportable adverse experience involves two or more suspect products from the same898
applicant, only one FDA Form 3500A should be completed. The FDA Form 3500A should899
reference only one manufacturer report number. The report should be submitted to the900
NDA, ANDA, or BLA considered most suspect by the initial reporter. If each product is901
equally suspect, the report should be submitted to the product first in alphabetical order.902
The adverse experience should also be reported in the narrative summary section of the903
periodic report for the other product(s).904
905
However, if one suspect product is a licensed non-vaccine biologic and the other is a906
licensed vaccine, separate reporting forms should be submitted. An FDA Form 3500A907
should be used for the licensed non-vaccine biologic and a VAERS form should be used908
for the licensed vaccine.909
910
If a reportable adverse experience involves two or more suspect products and two or more911
applicants, an applicant may choose to submit an FDA Form 3500A to the FDA on the912
adverse experience that describes detailed information including the product(s) from the913
20
The requirements for prescription drugs marketed for human use without an approved application can be
found in ' 310.305.

Draft — Not for Implementation
G:\4177dft.doc
23
other applicant. In such a case, the other applicant should receive a copy of the FDA Form914
3500A including its manufacturer report number so that the other applicant can reference915
this report when providing any relevant followup information to the FDA. The other applicant916
should not submit to the FDA information originally submitted to the Agency by the first917
applicant.918
919
L. Suspect Drugs with Multiple NDAs or ANDAs by the Same Applicant920
921
A drug substance can be the subject of more than one approved NDA or ANDA. If an922
applicant receives a report for a drug and the specific application is identifiable, the report923
should be submitted to that application. However, if a drug substance has more than one924
application and it cannot be determined which of the approved applications is involved, the925
report should be submitted to the application for the drug product that was approved first926
and that has the same general route of administration as the suspect drug substance. This927
would usually be the application with the lowest number.928
929
M. Two or More Marketers of a Product930
931
If two or more companies that co-market a specific drug product have an approved NDA932
for the product, one of the companies should be identified as having primary responsibility933
for reporting adverse experiences for the drug product to the FDA to avoid duplicative934
reporting of adverse experiences. This would also be true for two or more companies that935
co-market a specific biological product and have an approved BLA for the product.936
937
N. Unapproved Indications938
939
An adverse experience associated with the use of a product for an unapproved indication940
should be reported to the FDA as is required for any other spontaneously reported adverse941
experience occurring in the United States (e.g., 15-day report for a serious, unexpected942
adverse experience or periodic report for a nonserious, unexpected adverse experience). 943
However, a lack of effect report for an unapproved indication should not be reported on an944
FDA Form 3500A. Instead, such information should be included in the narrative summary945
section of a periodic report.946
947
O. Product Interactions948
949
If an applicant receives a report identified as a product interaction, each of the products950
should be identified as a suspect product in item C1 of FDA Form 3500A.951
952
P. Reports from the FDA
21
953
954
21
The requirements for submitting reports received from the FDA can be found in '' 310.305(c)(5),
314.80(b), and 600.80(b).
Draft — Not for Implementation
G:\4177dft.doc
24
Sometimes FDA forwards individual case safety reports (i.e., FDA Form 3500As) to955
applicants. For example, applicants can participate in the FDA’s MedWatch-to-956
Manufacturer Program. This program is designed to expedite transmission from the FDA957
to applicants participating in the program cases of serious adverse experiences reported958
directly to the FDA voluntarily by initial reporters (e.g., health care professionals,959
consumers). Details of the program can be found on the Internet at960
www.fda.gov/medwatch/report/mmp.htm.961
962
Applicants that receive individual case safety reports from FDA are not required to963
resubmit them to the Agency. However, followup information to these initial reports must964
be submitted to the FDA (see section V.C in this guidance).965
966
Q. Product Defects967
968
If a product defect results in an adverse experience, the adverse experience should be969
reported as any other spontaneously reported adverse experience occurring in the United970
States (e.g., 15-day report for a serious, unexpected adverse experience or periodic report971
for a nonserious, unexpected adverse experience). 972
973
R. Reporting Ambiguities974
975
In some cases, it may be difficult to interpret specific criteria used for reporting. Examples976
include determining whether an adverse experience is expected or unexpected or whether977
a patient is identifiable or not. For these and any other ambiguities, the applicant should978
use a conservative approach and err on the side of reporting the adverse experience to the979
FDA. Thus, if there is doubt, consider an adverse experience to be unexpected, consider980
a patient to be identifiable, and so on.981
982
983
VII. CODING OF ADVERSE EXPERIENCES IN INDIVIDUAL CASE SAFETY984
REPORTS985
986
Companies currently use a variety of medical terminologies to code adverse experiences987
in individual case safety reports (e.g., COSTART, WHOART, MedDRA). At this time, the988
FDA will accept adverse experiences coded with any of these terminologies. However, as989
recommended by ICH, the Agency encourages companies to use MedDRA for this990
purpose and as indicated in the FDA’s advanced notice of proposed rulemaking on this991
topic (63 FR 59746; November 5, 1998), the Agency plans to propose to require use of992
MedDRA as the terminology for coding adverse experiences in individual case safety993
reports submitted to the FDA.994
995
Companies can license MedDRA from an international maintenance and support services996
organization (MSSO) (toll free number 877-258-8280 (703-345-7799 in Washington D.C.997
area), fax 703-345-7755, e-mail [email protected], Internet at998
www.meddramsso.com).999

Draft — Not for Implementation
G:\4177dft.doc
25
1000
1001
VIII. REPORTING FORMATS
22
1002
1003
Individual case safety reports of adverse experiences that occur domestically for marketed1004
human drugs and biological products, except vaccines, must be submitted to the FDA on1005
FDA Form 3500A; a VAERS form must be used for vaccines. Foreign adverse1006
experiences can be submitted either on FDA Form 3500A or, if preferred, on a CIOMS I1007
form. Foreign adverse experiences associated with the use of vaccines can be submitted1008
on either a VAERS form or, if preferred, a CIOMS I form. A separate FDA Form 3500A,1009
VAERS form, or CIOMS I form must be completed for each individual person experiencing1010
an adverse experience.1011
1012
The following paragraphs describe how to acquire or generate the various reporting forms1013
for individual case safety reports and how to obtain information on FDA’s pilot program for1014
electronic submission of these reports. This section also describes a suggested reporting1015
format for distribution reports for human biological products with approved BLAs.1016
1017
The following abbreviations should be used when specific information is not available for1018
an individual case safety report or distribution report:1019
1020
• NA for not applicable1021
• NI for no information at this time (but may be available later)1022
• UNK for unknown1023
1024
A. FDA Form 3500A1025
1026
See Appendix C of this guidance for a copy of the form.
23
1027
1028
1. Copies of the FDA Form 3500A can be obtained in the following ways:1029
1030
• From the Internet at www.fda.gov/medwatch/report/mfg.htm. Print the1031
form or download it as a PDF file. Form software can also be1032
downloaded and used to complete the forms using a personal1033
computer. Completed forms should be mailed to the FDA because1034
this software does not permit electronic submission of reports. The1035
software is also available on disk. For a copy of the disk, call 1-800-1036
FDA-1088 or send an electronic request via the MedWatch comment1037
page (www.fda.gov/medwatch/report/mfg.htm). Note: this software1038
22
The requirements for reporting formats can be found in '' 310.305(d), 314.80(f) and 600.80(f).
23
Instructions for completing FDA Form 3500A are available on the Internet at
www.fda.gov/medwatch/report/instruc.htm.
Draft — Not for Implementation
G:\4177dft.doc
26
contains both FDA Form 3500 for voluntary reporting and FDA Form1039
3500A for mandatory reporting.1040
1041
• By Fax - Call 1-800-FDA-1088 and make the following selections:1042
1043
Press 1 (health professional)1044
Press 2 (obtain a copy of a form)1045
Press 4 (obtain fax of FDA Form 3500A).1046
1047
• By Mail - Up to 10 copies of FDA Form 3500A can be obtained from:1048
1049
Office of Post-marketing Drug Risk Assessment1050
Center for Drug Evaluation and Research (HFD-400)1051
Food and Drug Administration1052
5600 Fishers Lane, Room 15B-311053
Rockville, MD 208571054
1055
OR1056
1057
Office of Biostatistics and Epidemiology1058
Center for Biologics Evaluation and Research (HFM-210)1059
Adverse Experience Reporting1060
1401 Rockville Pike1061
Rockville, MD 20852-14481062
1063
Additional copies can be obtained from:1064
1065
Consolidated Forms and Publications Distribution Center1066
Washington Commerce Center1067
3222 Hubbard Rd.1068
Landover, MD 207851069
1070
2. Copies can be created by:1071
1072
• Photocopying a blank FDA Form 3500A1073
1074
• Producing a computer-generated facsimile of FDA Form 3500A1075
1076
In place of using the preprinted forms, a computer-generated facsimile of1077
FDA Form 3500A can be used after approval, in writing, by FDA (''1078
310.305(d)(3), 314.80(f)(3) and 600.80(f)(3)). This computer-generated1079
facsimile of FDA Form 3500A should:1080
1081
a. Contain all the elements (i.e., 2-column format, sections, blocks, titles,1082
descriptors within blocks, text for disclaimer) of FDA Form 3500A in1083
Draft — Not for Implementation
G:\4177dft.doc
27
the identical enumerated sequence of the form, except as otherwise1084
noted. For reports in which no suspect medical device is involved, the1085
box Section D. Suspect Medical Device on the front page of FDA1086
Form 3500A can be replaced with the box Section G. All1087
Manufacturers located on the back page of the form. This would allow1088
reporters of adverse experiences for drug and biological products to1089
use a one-page form for reporting. See Appendix F of this guidance1090
for a sample of a one-page FDA Form 3500A).1091
1092
b. Have, at least, a 1/4" margin around the entire form so that1093
information is not lost during scanning, copying or faxing of the1094
document (the left-hand margin may be increased up to 2" to permit1095
binding (e.g., hole-punching) of the form) (all other margins have to1096
continue to be at least 1/4").1097
1098
c. Include the name of the company centered on the top of the front1099
page.1100
1101
d. Include in the lower left corner of the front page the phrase 3500A1102
Facsimile instead of the phrase FDA Form 3500A (date of form [e.g.,1103
6/93]).1104
1105
e. Include in the upper right corner of the front page above the FDA Use1106
Only box the phrase FDA Facsimile Approval: [include date of1107
approval by FDA], instead of the phrase See OMB statement on1108
reverse.1109
1110
f. Have the data and text contained within the boxes on a computer-1111
generated FDA Form 3500A conform to the following specifications:1112
1113
• The font size should not be less than 10 point.1114
1115
• A font type should be selected that is easy to read (e.g., CG1116
Times, Arial) and not condensed. The form may be copied or1117
faxed multiple times. For visual contrast, the font type used for1118
the data and text should, if possible, be different from the font1119
type used to create the FDA Form 3500A.1120
1121
• Have all data and text contained within each of the boxes (e.g.,1122
a box marked with an Ax@ should be centered within the box,1123
and narratives should include margins so that letters are not1124
obscured or made ambiguous by lines defining a box.).1125
1126

Draft — Not for Implementation
G:\4177dft.doc
28
• Have the phrase continued included at the end of each field1127
that has additional information continued onto another page.1128
1129
g. Have continuation pages containing additional information for1130
narrative entries conform to the following specifications:1131
1132
• Each page should be identified as Page _ of _.1133
1134
• Each page should include the manufacturer report number in1135
the upper right corner.1136
1137
• Each page should include the name of the company in the1138
upper right corner.1139
1140
• The section and block number (e.g., Block B5) for each1141
narrative entry should be included.1142
1143
For approval of computer-generated facsimiles of FDA Form 3500As, companies1144
should mail their requests along with two copies of the computer-generated1145
facsimile, a blank one and one with all the boxes completed with sample data/text,1146
to:1147
1148
Information Technology Staff1149
OPDRA/CDER/FDA Room 15B231150
HFD-4201151
5600 Fishers Lane1152
Rockville, MD 208571153
1154
Companies can contact the Information Technology Staff at 301-827-3223 to check1155
on the status of an approval request. Companies that are using a computer-1156
generated facsimile of FDA Form 3500A from a vendor that has already obtained1157
approval, in writing, from the FDA for the form do not have to submit another1158
approval request to the Agency (the vendor=s name and approval date should1159
appear in the upper right corner of the form). 1160
1161
B. VAERS Form for Vaccines1162
1163
See Appendix D of this guidance for a copy of the form.
24
1164
24
A guidance for industry entitled How to Complete the Vaccine Adverse Event Reporting System Form
(VAERS-1) (October 1999) is available on the Internet at www.fda.gov/cber/guidelines.htm or from the Office
of Communication, Training and Manufacturers Assistance (HFM-40), CBER, 1401 Rockville Pike, Rockville,
MD 20852-1448, (Fax) 1-888-CBERFAX or 301-827-3844, (Voice Information) 1-800-835-4709 or 301-827-
1800.

Draft — Not for Implementation
G:\4177dft.doc
29
1165
1. Copies of the VAERS form can be obtained by calling 1-800-822-7967.1166
1167
2. In place of using the preprinted forms, a computer-generated facsimile of the1168
VAERS form can be used after approval, in writing, by FDA (' 600.80(f)(3)). 1169
To request approval of a computer-generated facsimile of a VAERS form, a1170
printed copy with data to illustrate how each data field will be reported should1171
be submitted to:1172
1173
Office of Biostatistics and Epidemiology (HFM-210)1174
Center for Biologics Evaluation and Research, FDA1175
1401 Rockville Pike1176
Rockville, MD 20852-14481177
1178
C. CIOMS I Form for Foreign Adverse Experiences1179
1180
CIOMS, working with several member nations and industry, has developed a format for1181
international adverse experience reporting (CIOMS I form) (see Appendix E of this1182
guidance). Applicants can use an FDA Form 3500A or, if preferred, a CIOMS I form for1183
submission of 15-day reports of foreign adverse experiences to the FDA. Applicants1184
cannot use a CIOMS I form for submissions of adverse experiences that occur within the1185
United States. For these adverse experiences, an FDA Form 3500A must be used.1186
1187
D. Distribution Reports for Biological Products Including Vaccines1188
1189
This section on distribution reports only applies to human biological products with1190
approved BLAs. Under ' 600.81, distribution reports must include the bulk lot, fill lot, and1191
label lot numbers for the total number of dosage units of each strength or potency1192
distributed (e.g., 50,000 per 10-milliliter vials), labeled date of expiration, and date of1193
distribution of fill lot or label lot. The report must also include information about any1194
significant amount of a fill lot or label lot that may have been returned.1195
1196
The regulations do not specify a reporting form or format for distribution reports. One1197
suggested report format is shown here:1198
1199
1200
Product name, strength 1201
Biologics License No. Product Code 1202
1203
Bulk Lot
No.
Fill Lot
No.
Label Lot
No.
Expiration
Date
Distribution
Date
No. of Doses
Distributed
No. of Doses
Returned
1204
Draft — Not for Implementation
G:\4177dft.doc
30
1205
If there is more than one distribution date for a lot, the report should include each1206
distribution date and the number of doses distributed. When reporting returned doses, the1207
number of doses distributed should not be repeated.1208
1209
For vaccines, if available, distribution of doses can be reported by public, private, or1210
military sectors.1211
1212
E. Electronic Submissions1213
1214
The FDA is in the process of developing a system for electronic submission of1215
postmarketing safety reports. At this time, applicants can submit, under a pilot program,1216
certain individual case safety reports electronically. Details of this pilot program are1217
available on the Internet at www.fda.gov/cder/aerssub. The Agency also plans to have a1218
system for electronic submission of distribution reports for biological products including1219
vaccines in the near future. 1220
1221
1222
IX. HOW AND WHERE TO SUBMIT POSTMARKETING SAFETY REPORTS1223
1224
All submissions should be legible and typewritten with a minimum acceptable font size of1225
10 point. Legible photostatic copies can be submitted. However, visual contrast should be1226
adequate to ensure clear readable archival images. The applicant must submit one or two1227
copies of each safety report as specified in this section unless a waiver is granted1228
permitting a different number of copies (see section XI in this guidance).1229
1230
A. Human Drug Products1231
1232
1. For prescription drugs marketed for human use without an approved NDA1233
or ANDA, postmarketing 15-day reports (initial and followup) should be sent1234
as single copies to:1235
1236
Office of Post-marketing Drug Risk Assessment (HFD-400)1237
Center for Drug Evaluation and Research1238
Food and Drug Administration1239
5600 Fishers Lane1240
Rockville, MD 208571241
1242
2. For drugs with approved ANDAs, postmarketing 15-day reports, (initial and1243
followup), and periodic reports should be sent as single copies to:1244
1245
Office of Post-marketing Drug Risk Assessment (HFD-400)1246
Center for Drug Evaluation and Research1247
Food and Drug Administration1248
5600 Fishers Lane1249
Draft — Not for Implementation
G:\4177dft.doc
31
Rockville, MD 208571250
1251
3. For drugs with approved NDAs, postmarketing 15-day reports (initial and1252
followup), and periodic reports should be sent in duplicate to:1253
1254
Food and Drug Administration1255
Central Document Room1256
12229 Wilkins Ave.1257
Rockville, MD 208521258
1259
B. Human Biological Products and Vaccines1260
1261
1. For vaccines, postmarketing 15-day reports (initial and followup), and1262
periodic reports should be sent in duplicate to:1263
1264
VAERS1265
P.O. Box 11001266
Rockville, MD 20849-11001267
1268
2. For biological products other than vaccines, postmarketing 15-day reports1269
(initial and followup) and periodic reports should be sent in duplicate to:1270
1271
Office of Biostatistics and Epidemiology (HFM-210)1272
Center for Biologics Evaluation and Research, FDA1273
Adverse Experience Reporting1274
1401 Rockville Pike1275
Rockville, MD 20852-14481276
1277
3. For all biological products and vaccines, distribution reports (' 600.81)1278
should be sent in duplicate to:1279
1280
Office of Biostatistics and Epidemiology (HFM-210)1281
Center for Biologics Evaluation and Research, FDA1282
Distribution Reports1283
1401 Rockville Pike1284
Rockville, MD 20852-14481285
1286
1287
X. WRITTEN PROCEDURES FOR POSTMARKETING SAFETY REPORTING1288
1289
Each applicant must develop written standard operating procedures for the surveillance,1290
receipt, evaluation, and reporting of adverse experiences to the FDA ('' 310.305(a),1291
314.80(b) and 600.80(b)). The FDA will consider an applicant responsible for information1292
known to its employees, affiliates, and contractors. For this purpose, applicants should1293
Draft — Not for Implementation
G:\4177dft.doc
32
develop procedures that allow for expedited handling of adverse experience reports. 1294
Records of due diligence should be maintained. This applies to surveillance and1295
processing for both domestic and foreign reports of adverse experiences.1296
1297
1298
XI. REQUESTS FOR WAIVERS TO POSTMARKETING SAFETY REPORTING1299
REQUIREMENTS1300
1301
Under '' 314.90(a) and 600.90(a), applicants may ask the FDA to waive any1302
postmarketing safety reporting requirement that applies to the applicant under '' 314.801303
and 600.80. The following paragraphs discuss certain postmarketing periodic safety1304
reporting requirements for which the FDA is currently granting waivers.1305
1306
A. Submission of FDA Form 3500A for Nonserious, Expected Adverse1307
Experiences 1308
1309
Applicants are encouraged to request a waiver for submission of FDA Form 3500As for1310
individual case safety reports of nonserious, expected adverse experiences that, at a1311
minimum, contain the four basic elements (see section IV.B in this guidance). In such1312
cases, applicants should maintain records of these nonserious, expected adverse1313
experiences in their corporate drug or biological product safety files. The FDA may1314
request that an applicant submit to the Agency FDA Form 3500As of one or more of these1315
adverse experiences. The agency would expect these forms to be submitted within 51316
calendar days after receipt of the request.1317
1318
Applicants who obtain a waiver for the requirement to submit individual case safety reports1319
of nonserious, expected adverse experiences would still be expected to submit information1320
on these adverse experiences to the FDA in the summary tabulations section of1321
postmarketing periodic reports (see section V.B.2.a in this guidance).1322
1323
At this time, the FDA does not intend to grant waiver requests for new biological molecular1324
entities within one year of licensure or for blood products, plasma derivatives, or vaccines. 1325
The Agency believes that it is important to continue periodic review of all individual case1326
safety reports of adverse experiences for these products to identify safety problems due to1327
lot-to-lot variations and also to monitor the safety of newly approved biological products.1328
1329
B. Submission of PSUR format for the Periodic Report1330
1331
Applicants can request a waiver of the requirement to submit postmarketing periodic1332
safety reports in the format described in the regulations. Instead, applicants can prepare1333
these reports using the PSUR (Periodic Safety Update Report) format described in the1334
ICH E2C guidance. In addition, the Agency recommends the following:1335
1336
• If all dosage forms and formulations for the active substance, as well as1337
indications, are combined in one PSUR, this information should be1338

Draft — Not for Implementation
G:\4177dft.doc
33
separated into specific sections of the report when such separation is1339
appropriate to accurately portray the safety profile of the specific dosage1340
forms. For example, one should not combine information from ophthalmic1341
drop dosage forms and solid oral dosage forms. One copy of the PSUR1342
should be submitted for each approved NDA or ANDA whose product is1343
covered in the PSUR as well as an additional copy for review by the1344
postmarketing pharmacovigilance office.1345
1346
• Copies of the FDA Form 3500A or VAERS form that are required by the1347
regulations must be included.
25
These forms should be included with the1348
PSUR as an appendix. You can request a waiver for submission of certain1349
nonserious, expected adverse experiences on an FDA Form 3500A as1350
described in the previous section.1351
1352
• A summary tabulation should be included as an appendix listing all1353
spontaneously reported U.S. individual case safety reports from consumers if1354
such cases are not already included in the PSUR. Summary tabulations1355
should be presented by body system of all adverse experience terms and1356
counts of occurrences and be segregated by type (i.e., serious/unexpected;1357
serious/expected; nonserious/unexpected; and nonserious/expected),1358
1359
• A narrative should be included as an appendix that references the changes,1360
if any, to the approved U.S. labeling for the dosage forms covered by the1361
PSUR based on new information in the PSUR. A copy of the most recently1362
approved U.S. labeling for the product(s) covered by the PSUR should be1363
included.1364
1365
C. Submission Date and Frequency for PSUR Reports1366
1367
Applicants can request a waiver to submit PSURs to the FDA based on the month and day1368
of the international birth date of the product instead of the month and day of the anniversary1369
date of U.S. approval of the product.
26
The waiver request should specify that these1370
PSURs would be submitted to the FDA within 60 calendar days of the data lock point (i.e.,1371
month and day of the international birth date of the product or any other day agreed on by1372
the applicant and the FDA).
27
1373
1374
Applicants can also request a waiver to submit PSURs to the FDA at a frequency other1375
than those required under § § 314.80(c)(2)(i) and 600.80(c)(2)(i).1376
1377
25
See '' 314.80(c)(2)(ii)(b) and 600.80(c)(2)(ii)(B).
26
The international birth date for a product is the date the first regulatory authority in the world approved the
first marketing application for a human drug product containing the drug substance or a human biological
product.
27
The data lock point is the date designated as the cut-off date for data to be included in a PSUR.
Draft — Not for Implementation
G:\4177dft.doc
34
D. How and Where to Submit Waiver Requests1378
1379
1. Marketed human drug products1380
1381
For waivers under ' 314.90(a), applicants should submit a written waiver request1382
(include the product’s name(s), date(s) of U.S. approval, and the application1383
number(s)) to:1384
1385
Director1386
Office of Post-Marketing Drug Risk Assessment1387
Center for Drug Evaluation and Research1388
Food and Drug Administration1389
5600 Fishers Lane, HFD-4001390
Rockville, MD 208571391
1392
2. Licensed biological products1393
1394
For waivers under ' 600.90(a), applicants should submit a written waiver request1395
(include the product name(s), date(s) of U.S. approval, and the application1396
number(s)) to:1397
1398
Director1399
Office of Biostatistics & Epidemiology1400
Center for Biologics Evaluation and Research1401
Food and Drug Administration1402
140l Rockville Pike, HFM-2201403
Rockville, MD 20852-14481404
1405
1406
XII. VALIDATION OF ADVERSE EXPERIENCE COMPUTER SYSTEMS1407
1408
If an electronic record of an adverse experience is created, modified, maintained,1409
archived, retrieved, or transmitted, the applicant is required, among other things, to employ1410
procedures to ensure that records are trustworthy, reliable, and consistent with FDA's1411
ability to promote and protect public health (21 CFR part 11). Those procedures must1412
include validation of systems to ensure accuracy, reliability, consistent intended1413
performance, and the ability to discern invalid or altered records.1414
Draft — Not for Implementation
G:\4177dft.doc
35
APPENDIX A: GLOSSARY1415
1416
Adverse Experience - Any adverse event associated with the use of a drug or biological1417
product in humans, whether or not considered product-related, including the following: An1418
adverse event occurring in the course of the use of a drug product in professional practice;1419
an adverse event occurring from drug overdose whether accidental or intentional; an1420
adverse event occurring from drug abuse; an adverse event occurring from drug1421
withdrawal; and any failure of expected pharmacological action. Reporting an adverse1422
experience does not necessarily reflect a conclusion by the applicant or the FDA that the1423
product caused or contributed to the adverse experience. Adverse experience is1424
synonymous with adverse drug experience, adverse biological experience, adverse1425
product experience, and adverse event. 1426
1427
Affiliate - Any individual or entity related by employment or organizational structure to the1428
applicant, including all subsidiaries, whether domestic or foreign.1429
1430
Applicant - An individual or entity who holds the new drug application (NDA), abbreviated1431
new drug application (ANDA), or the biologics license application (BLA). For purposes of1432
this guidance, this term includes any person whose name appears on the label of a1433
marketed drug or licensed biological product as its manufacturer, packer, distributor,1434
shared manufacturer, joint manufacturer, or any participant involved in divided1435
manufacturing.1436
1437
Causality Assessment - Determination of whether there is a reasonable possibility that1438
the product is etiologically related to the adverse experience. Causality assessment1439
includes, for example, assessment of temporal relationships, dechallenge/rechallenge1440
information, association with (or lack of association with) underlying disease, presence (or1441
absence) of a more likely cause, and physiologic plausibility.1442
1443
Challenge - Administration of a suspect product by any route.1444
1445
Dechallenge - Withdrawal of a suspect product from a patient's therapeutic1446
regimen.1447
1448
Negative Dechallenge - Continued presence of an adverse experience after1449
withdrawal of the suspect product.1450
1451
Positive Dechallenge - Partial or complete disappearance of an adverse1452
experience after withdrawal of the suspect product.1453
1454
Rechallenge - Reintroduction of a suspect product suspected of having caused an1455
adverse experience following a positive dechallenge.1456
1457
Draft — Not for Implementation
G:\4177dft.doc
36
Negative Rechallenge - Failure of the product, when reintroduced, to produce1458
signs or symptoms similar to those observed when the suspect product was1459
previously introduced.1460
1461
Positive Rechallenge - Reoccurrence of similar signs and symptoms upon1462
reintroduction of the suspect product.1463
1464
Disability - A substantial disruption in one's ability to conduct normal life functions.1465
1466
Expected Adverse Experience - Adverse experience listed in the current FDA-approved1467
labeling for the drug or licensed biological product. This would include any section of the1468
labeling that refers to adverse experience information.1469
1470
Initial Reporter - The original source of information concerning an adverse experience1471
(e.g., consumer, healthcare professional).1472
1473
Life-threatening Adverse Experience - An adverse experience that, in the view of the1474
initial reporter, places the patient at immediate risk of death from the adverse experience1475
as it occurred. It does not include an adverse experience that, had it occurred in a more1476
severe form, might have caused death.1477
1478
Serious Adverse Experience - An adverse experience occurring at any dose that results1479
in any of the following outcomes:1480
1481
• Death1482
1483
• Life-threatening adverse experience1484
1485
• Initial inpatient hospitalization1486
1487
• Prolongation of hospitalization1488
1489
• Significant or persistent disability/incapacity1490
1491
• Congenital anomaly/birth defect (including that occurring in a fetus);1492
1493
• Important medical events, based upon appropriate medical judgment, that may1494
jeopardize the patient or subject and may require medical or surgical intervention to1495
prevent one of the outcomes listed above.1496
1497
Spontaneous Report - A communication from an individual (e.g. health care1498
professional, consumer) to a company or regulatory authority that describes a suspected1499
adverse experience. It does not include cases identified from information solicited by the1500
applicant such as individual cases or findings derived from a study.1501
1502
Draft — Not for Implementation
G:\4177dft.doc
37
Study - Any organized data collection system (e.g., adverse experience information1503
derived from a clinical trial, patient registry including pregnancy registries). Reports from1504
company sponsored patient support programs and disease management programs should1505
be handled as if they were study reports and not as spontaneous reports.1506
1507
Suspect Product - Drug or biological product associated with an adverse experience as1508
determined by the initial reporter, regardless of the opinion of the applicant.1509
1510
Unexpected Adverse Experience - Adverse experience not included in any section of1511
the current FDA-approved labeling for the drug or licensed biological product. This1512
includes an adverse experience that may differ from a labeled adverse experience1513
because of greater severity or specificity (e.g., abnormal liver function versus hepatic1514
necrosis). Adverse experiences listed as occurring with a class of drugs or biological1515
products but not specifically mentioned with a particular drug or biological product are1516
considered unexpected (e.g., rash with antibiotic X would be unexpected if the labeling1517
said "rash may be associated with antibiotics"). This is because the labeling does not1518
specifically state "rash is associated with antibiotic X." Reports of death from an adverse1519
experience are considered unexpected unless the possibility of a fatal outcome from that1520
adverse experience is stated in the labeling.1521
1522
Draft — Not for Implementation
G:\4177dft.doc
38
APPENDIX B: REPORT CHECKLIST1523
1524
Before mailing your postmarketing safety reports to the FDA, you should make sure that1525
the following questions have been addressed:1526
1527
A. For All FDA Form 3500A Reports1528
1529
l. Have you completed a separate FDA Form 3500A for each patient?1530
1531
2. Have you included the manufacturer report number in item G9 on FDA Form1532
3500A? (Note: For followup reports, this number should be identical to the1533
manufacturer report number on the initial report.)1534
1535
3. Have you clearly marked the report "Periodic" or "15-Day" as appropriate in1536
item G7 on FDA Form 3500A?1537
1538
4. Have you clearly marked the report "Initial" or "Followup" as appropriate in1539
item G7 on FDA Form 3500A? Do not package and send a 15-day followup1540
report with a non-15 day followup report.1541
1542
5. Have you included the name, address, and telephone number of the initial1543
reporter in item E1 on FDA Form 3500A?1544
1545
6. Have you left all the boxes in item B2 of the FDA Form 3500A blank for a1546
nonserious adverse experience? A box should only be checked in item B2 if1547
the outcome for the adverse experience is serious.1548
1549
7. Have you included all relevant attachments and eliminated unnecessary1550
attachments?1551
1552
Attachments can include copies of:1553
1554
• hospital discharge summaries1555
• autopsy/biopsy reports1556
• death certificates1557
• relevant office visit notes1558
• summaries of relevant laboratory tests and other diagnostic1559
procedures, particularly pre- and post-drug values.1560
1561
Each page of an attachment should identify the manufacturer report number (i.e.,1562
reported in item G9 on FDA Form 3500A) for that case.1563
1564
In general, attachments should not include:1565
1566
• lengthy legal records1567
Draft — Not for Implementation
G:\4177dft.doc
39
• complete medical records1568
1569
1570
8. If two or more products produced by your company were suspected by the1571
initial reporter:1572
1573
• Have you completed only one FDA Form 3500A? (You should not1574
prepare more than one FDA Form 3500A even if more than one of1575
the suspect products was produced by your company.)1576
1577
• Have you identified all the suspect products in item C1 on FDA Form1578
3500A?1579
1580
• Have you indicated on FDA Form 3500A the product considered1581
most suspect by the initial reporter and prepared the report1582
accordingly? (If the initial reporter ranked them equally, you should1583
submit an FDA Form 3500A to the file of the first suspect product in1584
alphabetical order. You should list the adverse experience(s) for each1585
of the other suspected product(s) in the narrative summary section of1586
the periodic report.)1587
1588
9. Have you completed an FDA Form 3500A for another applicant's drug? 1589
(You should send the report to the FDA if the applicant of the suspect product1590
is unknown or the report is for a serious, unexpected adverse experience1591
occurring during the conduct of a study. For all other cases, you should send1592
the report to the applicant holder of the suspect drug and not to the FDA.)1593
1594
B. For 15-Day Reports1595
1596
1. Have you clearly marked "15-Day Report" in item G7 on the FDA Form1597
3500A?1598
1599
2. Have you packaged the 15-day report (FDA Form 3500A initial or followup)1600
separately? (Do not package and send an initial 15-day report with a 15-day1601
followup report. You should not submit copies of 15-day reports with a1602
periodic report.)1603
1604
3. Have you submitted the report in duplicate? (Exceptions: for prescription1605
drugs marketed for human use without an approved application and for drugs1606
with approved ANDAs, only a single copy should be sent.)1607
1608
4. Have you clearly marked the outside mailing envelope "15-Day Report”?1609
Draft — Not for Implementation
G:\4177dft.doc
40
1610
C. For Periodic Reports1611
1612
1. Have you included the four types of information required for a periodic report1613
and clearly separated the four sections with marked tabs?1614
1615
2. Have you submitted the report in duplicate? (Exception: For drugs with1616
approved ANDAs, only a single copy should be sent).1617
1618
3. Have you eliminated all unnecessary attachments to FDA Form 3500As?1619
1620
D. For Followup Reports1621
1622
l. Have you included the manufacturer report number in item G9 on FDA Form1623
3500A? (Note: this number should be identical to the manufacturer report1624
number on the initial report).1625
1626
2. Have you marked Afollowup@ in item G7 on FDA Form 3500A and indicated1627
what number followup report it is?1628
1629
1630