Name: ___________
AP Chemistry
Gases Practice Problems
Multiple Choice
Identify the letter of the choice that best completes the statement or answers the question. You
____ 1. At standard temperature and pressure, a 0.50 mol sample of H
2
gas and a separate 1.0 mol sample of O
2
gas
have the same
a.
average molecular kinetic energy
c.
volume
b.
average molecular speed
d.
effusion rate
____ 2. A rigid metal tank contains oxygen gas. Which of the following applies to the gas in the tank when
additional oxygen is added at constant temperature?
a.
The volume of the gas increases.
b.
The pressure of the gas decreases.
c.
The average speed of the gas molecules remains the same.
d.
The total number of gas molecules remains the same.
____ 3. What volume of O2(g) is required to react with excess CS2(!) to produce 4.0 L of CO2(g)? (Assume all
gases are measured at 0°C and 1 atm.)
CS2(!) + 3 O2(g) ® CO2(g) + 2 SO2(g)
a.
12 L
c.
1/3 ´ 22.4 L
b.
22.4 L
d.
2 ´ 22.4 L
____ 4. A hydrocarbon gas with an empirical formula CH
2
has a density of 1.88 grams per liter at 0
o
C and 1.00
atmosphere. Which of the following is a possible molecular formula for the hydrocarbon?
a.
CH
2
c.
C
3
H
6
b.
C
2
H
4
d.
C
4
H
8
____ 5. A 2 L container will hold about 4 g of which of the following gases at 0°C and 1 atm?
a.
SO2
c.
CO2
b.
N2
d.
C4H8
____ 6. Under which conditions will a real gas behave most like an ideal gas?
a.
high pressure and high temperature
c.
low volume and high temperature
b.
low pressure and low temperature
d.
low pressure and high temperature
____ 7. When a sample of oxygen gas in a closed container of constant volume is heated until its absolute temperature
is doubled, which of the following is also doubled?
a.
The density of the gas
b.
The pressure of the gas
c.
The average velocity of the gas molecules
d.
The number of molecules per cm
3

____ 8. Consider the graph below, which shows the speed distribution of a sample of gas molecules at four different
temperatures.
Which of the following correctly sequences the curves in order of increasing temperature?
a.
A, C, B, D
c.
A, B, C, D
b.
D, B, C, A
d.
D, C, B, A
____ 9. Equal masses of three different ideal gases, X, Y, and Z, are mixed in a sealed rigid container. If the
temperature of the system remains constant, which of the following statements about the partial
pressure of gas X is correct?
a.
It is equal to
1
/
3
the total pressure
b.
It depends on the intermolecular forces of attraction between molecules of X, Y,
and Z.
c.
It depends on the relative molecular masses of X, Y, and Z.
d.
It depends on the average distance traveled between molecular collisions.
____ 10. A flask contains 0.25 mole of SO2(g), 0.50 mole of CH4(g), and 0.50 mole of O2(g). The total pressure
of the gases in the flask is 800 mm Hg. What is the partial pressure of the SO2(g) in the flask?
a.
800 mm Hg
c.
250 mm Hg
b.
600 mm Hg
d.
160 mm Hg
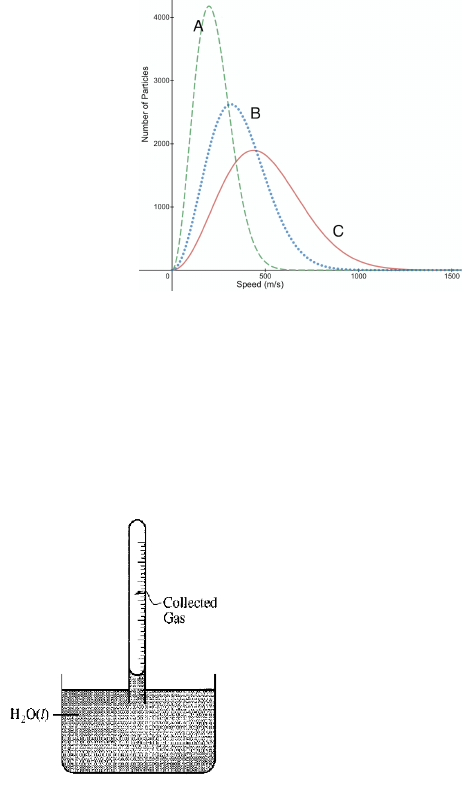
____ 11. Consider the graph below, which shows the speed distribution of three different pure substances in the gas
phase at a temperature of 566
o
C. Which of the following statements accurately describes the molar masses of
the substances and provides the correct explanation?
a. Substance A has the highest molar mass, because Substance A has the lowest average speed.
b. Substance C has the highest molar mass, because Substance C has the highest average speed.
c. Substance A has the lowest molar mass, because Substance A has the lowest average speed.
d. Substances A, B and C all have the same molar mass.
____ 12. In a laboratory experiment, H
2
(g) is collected over water in a gas-collection tube as shown in the diagram
below.
The temperature of the water is 21
o
C and the atmospheric pressure in the laboratory is measured to be 772
torr. Before measuring the volume of gas collected in the tube, what step, if any, must be taken to make it
possible to determine the total gas pressure inside the tube?
a.
Tilt the tube to the side enough to let some air in to break the partial vacuum in the tube.
b.
Lift the tube upward until it is just barely immersed in the water
c.
Move the tube downward until the water level is the same inside and outside the tube.
d.
Adjust the temperature of the water to 25
o
C.

Free Response Questions
2003 B
A rigid 5.00 L cylinder contains 24.5 g of N
2
(g) and 28.0 g of O
2
(g)
13. a). Calculate the total pressure, in atm, of the gas mixture in the cylinder at 298 K
b) The temperature of the gas mixture in the cylinder is decreased to 280 K. Calculate each of the
following.
(i) The mole fraction of N
2
(g) in the cylinder.
(ii) The partial pressure, in atm, of N
2
(g) in the cylinder.
.
c) If the cylinder develops a pinhole-sized leak and some of the gaseous mixture escapes, would the
ratio
!
"($)
&
"($)
in the cylinder increase, decrease, or remain the same? Justify your answer.
1996
Represented below are five identical balloons, each filled to the same volume at 25°C and 1.0
atmosphere pressure with the pure gases indicated.
14. a) Which balloon contains the greatest mass of gas? Explain.
b) Which balloon contains the gas that would be expected to deviate most from the behavior of an
ideal gas? Explain your answer.
c) Compare the average kinetic energies of the gas molecules in the balloons. Explain.
d) Twelve hours after being filled, all the balloons have decreased in size. Predict which balloon
will be the smallest. Explain your reasoning.
15. When NH
3
gas is introduced at one end of a long tube while HCl gas is introduced simultaneously at
the other end, a ring of white ammonium chloride is observed to form in the tube after a few
minutes. This ring is closer to the HCl end of the tube than the NH
3
end. Explain this observation in
terms of molecular motion. (1993, modified)

1994 B
A student collected a sample of hydrogen gas by the displacement of water as shown by the diagram
above. The relevant data are given in the following table.
GAS SAMPLE DATA
Volume of sample
90.0 mL
Temperature
25°C
Atmospheric Pressure
745 mm Hg
Equilibrium Vapor Pressure
of H
2
O (25°C)
23.8 mm Hg
16. a) Calculate the number of moles of hydrogen gas collected.
b) Calculate the number of molecules of water vapor in the sample of gas.
c) Which of the two gases, H
2
or H
2
O, deviates more from ideal behavior? Explain your answer.
17. Two flasks are connected by a stopcock as shown below. The 5.0L flask contains CH
4
at a pressure of 3.0
atm, and the 1.0 L flask contains C
2
H
6
at a pressure of 0.55 atm. Calculate the total pressure of the system
after the stopcock is opened. Assume that the temperature remains constant. (2 pts)
In an experiment, a sample of an unknown, pure gaseous hydrocarbon was analyzed. Results showed that the
sample contained 6.000 g of carbon and 1.344 g of hydrogen. (1993B)
18. a) Determine the empirical formula of the hydrocarbon. (3 pts)

The density of the hydrocarbon at 25
o
C and 1.09 atm is 1.96 g L
-1
.
c) Calculate the molar mass of the hydrocarbon. (2 pts)
c) Determine the molecular formula of the hydrocarbon. (1 pt)
Answer the following questions about carbon monoxide, CO(g), and carbon dioxide, CO2(g). Assume
that both gases exhibit ideal behavior. (2004D)
19. a) Draw the complete Lewis structure (electron dot diagram) for the CO molecule and for the CO2
molecule. (2 pts)
b) Identify the geometry of the CO2 molecule. (1 pt)
c) A 1.0 mol sample of CO(g) is heated at constant pressure. On the graph below, sketch the expected
plot of volume verses temperature as the gas is heated. (1 pt)
d) Samples of CO(g) and CO2(g) are placed in 1 L containers at the conditions in the diagram below.

i) Indicate whether the average kinetic energy of the CO2 is greater than, equal to, or less than
the average kinetic energy of the CO(g) molecules. Explain your answer. (1 pt)
(ii) Indicate whether the average speed of the CO2(g) molecules is greater than, equal to or less
than the average speed of the CO(g) molecules. Explain your answer. (1 pt)
(iii) Indicate whether the number of CO2(g) molecules is greater than, equal, or less than the number
of CO(g) molecules. Justify your answer. (1 pt)
.

Gases Practice Problems
Answer Section
MULTIPLE CHOICE
1. ANS: A PTS: 1
2. ANS: C PTS: 1
3. ANS: A PTS: 1
4. ANS: C PTS: 1
5. ANS: C PTS: 1
6. ANS: D PTS: 1
7. ANS: B PTS: 1
8. ANS: C PTS: 1
9. ANS: C PTS: 1
10. ANS: D PTS: 1
11. ANS: A PTS: 1
12. ANS: C PTS: 1
PROBLEM
13. ANS:
24.5 g N
2
´ = 0.875 mol N
2
28.0 g O
2
´ = 0.875 mol O
2
P = =
= 8.56 atm
PTS: 1
b) :
(i) = 0.500 mole fraction N
2
(ii)
= 8.05 atm ´ mole fraction = 8.05 atm ´ 0.500
= 4.02 atm N
2
PTS: 1
d). decrease; since N
2
molecules are lighter than O
2
they have a higher velocity and will escape more
frequently (Graham’s Law), decreasing the amount of N
2
relative to O
2
PTS: 1
14. ANS:
a) CO
2
; according to Avogadro’s Hypothesis, they all contain the same number of particles,
therefore, the heaviest molecule, CO
2
(molar mass = 44), will have the greatest mass.
b) CO
2
; since they are all essentially non-polar, the largest intermolecular (London) force would be
greatest in the molecule/atom with the largest number of electrons.
c) all the same; at the same temperature all gases have the same kinetic energy.
d) He; it has the smallest size and has the greatest particulate speed and, therefore, it’s the easiest to
penetrate the wall and effuse.
PTS: 1
15. ANS:
The molecules of gas are in constant motion so the HCl and NH
3
diffuse along the tube. Where they
meet, NH
4
Cl(s) is formed. Since HCl has a higher molar mass, its velocity (average) is lower,
therefore, it doesn’t diffuse as fast as the NH
3
.
PTS: 1
16. ANS:
a) P
H2
= P
atm
- P
H2O
= (745 - 23.8) mm Hg) = 721.2 mm Hg
n = (PV)/(RT) = (721.2 mm Hg ´ 90.0 mL)/(62400 mm Hg.mL/mol.K ´ 298.15K)
= 3.49´10
-3
mol
b) n
H2O
= (23.8 mm Hg ´ 90.0 mL)/(62400 mm Hg.mL/mol.K ´ 298.15K) ´ 6.022´10
23
molecules/mol =
6.93´10
19
molecules
c) H
2
O deviates more from ideal behavior: (any one of these is sufficient)
(i) greater number of electrons = larger electron cloud = stronger London forces
(ii) it is a polar molecule with strong polar attraction, and attractions between molecules decreases the
observed gas pressure
(iii) it can interact via hydrogen bonds to other water molecules
(iv) H
2
O has a larger molecular volume than H
2
, so the available volume in the container is smaller
than expected for H
2
O, leading to a higher observed pressure.
17.
use P1V1 = P2V2
P
f
of methane = 2.5 atm; final pressure of ethane = 0.092 atm (1 pt for either)
P
tot
= 2.5 +0.092 atm = 2.6 atm (1 pt)

18.
a)C3H8
b) 43.9 g/mol
c). C3H8--must show work to verify/support answer to get the credit!
19. ANS:
:
(a)
b) linear
c)
d) (i) equal to; at the same temperature, all gas molecules have the same kinetic energy
(ii) less, since CO2 has a molar mass of 44 and CO has a mass of 28, the lighter molecule is faster at
the same temperature
(iii) less; Avogadro’s Hypothesis, equal volumes of gas at the same temperature and pressure contain
equal number of molecules. Since the pressure of CO2 is half the pressure of the CO, it must
contain half as many molecules.
