
Journal
of
Physiology
(1992),
455,
pp.
219-233
219
With
8
figures
Printed
in
Great
Britain
COHERENCE
BETWEEN
THE
SYMPATHETIC
DRIVES
TO
RELAXED
AND
CONTRACTING
MUSCLES
OF
DIFFERENT
LIMBS
OF
HUMAN
SUBJECTS
BY
B.
GUNNAR
WALLIN*,
DAVID
BURKE
AND
S.
C.
GANDEVIA
From
the
Department
of
Clinical
Neurophysiology,
The
Prince
Henry
and
Prince
of
Wales
Hospitals,
and
Prince
of
Wales
Medical
Research
Institute,
University
of
New
South
Wales,
Sydney
2036,
Australia
(Received
30
September
1991)
SUMMARY
1.
This
study
was
undertaken
to
quantify
the
simultaneous
sympathetic
drives
to
muscles
in
the
two
legs
of
human
subjects,
and
to
elucidate
the
extent
to
which
a
common
drive
determines
sympathetic
outflow
to
different
limbs
at
rest,
during
apnoea
and
during
voluntary
contractions.
2.
Sympathetic
efferent
activity
was
recorded
simultaneously
from
fascicles
of
both
peroneal
nerves,
innervating
the
pretibial
flexor
muscles.
At
rest
the
similarity
was
quantified
for
a
sample
of
records
by
manual
measurement
of
equivalent
bursts
in
the
two
recordings,
and
for
all
records
by
cross-correlation
and
power
spectral
analysis
of
the
two
recordings.
During
contractions,
only
the
latter
method
was
used.
3.
At
rest
the
correlation
coefficient
for
the
relationship
between
the
burst
amplitudes
for
the
two
recordings
was
0-72
(S.D.
0-1).
For
the
same
sequences,
the
computed
coherence
between
the
two
recordings
was
85-6
%
(S.D.
67
%)
at
the
cardiac
period.
There
was
a
statistically
significant
linear
relationship
between
these
two
measures
of
similarity,
and
this
was
stronger
when
data
from
sequences
recorded
during
apnoea
were
included
in
the
analysis.
At
rest
the
mean
difference
in
coherence
between
consecutive
sequences
with
no
intervening
manoeuvre
(apnoea,
contraction,
change
in
recording
site)
was
4-2
%
(S.D.
4.3
%).
In
only
two
of
forty-nine
such
instances
was
the
difference
in
coherence
>
10
%.
4.
Apnoea
at
end-expiration
increased
the
amplitude
and
frequency
of
sym-
pathetic
bursts
and
increased
the
similarity
between
the
two
recordings.
The
correlation
coefficients
increased
from
a
mean
of
0-72
at
rest
to 0-89
during
apnoea.
Coherence
increased
from
a
mean
of
82-1
%
at
rest
to
91-9
%
during
apnoea.
5.
On
the
right
side,
graded
voluntary
contractions
were
performed
at
5,
10,
20
or
30
%
maximal
force
using
the
muscle
innervated
by
the
fascicle
from
which
the
recording
was
made.
The
coherence
between
the
recordings
made
from
the
right
and
left
legs
decreased
by
>
10%
at
each
contraction
level.
Pooling
the
data
for
all
contractions,
there
was
a
significant
decrease
in
power
at
the
cardiac
frequency
in
the
*
Permanent
address:
Department
of
Clinical
Neurophysiology,
Sahlgrenska
Sjukhuset,
Goteborg,
Sweden.
MS
9780

B.
G.
WALLIN,
D.
BURKE
AND
S.
C.
GANDEVIA
sympathetic
recording
from
the
contracting
leg.
Contraction
of
a
synergist
or
antagonist
at
10%
maximum
produced
negligible
changes
in
coherence.
6.
It
is
concluded
that,
at
rest,
homologous
muscles
of
the
lower
limbs
are
subject
to
a
common
drive
and
that,
during
apnoea,
this
common
drive
can
dominate
the
sympathetic
outflow
to
the
virtual
exclusion
of
regional
drives.
During
voluntary
activity,
the
importance
of
this
common
drive
is
lessened,
presumably
because
of
regionally
specific
changes
involving
the
contracting
muscle.
The
data
suggest
that,
at
these
relatively
weak
contraction
levels,
voluntary
contraction
leads
to
a
reduction
in
sympathetic
outflow
to
the
contracting
muscle.
INTRODUCTION
At
rest,
there
is
a
remarkable
similarity
between
sympathetic
outflows
to
muscles
in
different
limbs
(Sundlbf
&
Wallin,
1977)
and
the
extent
of
the
similarity
suggests
that
muscle
sympathetic
activity
(MSA)
is
dominated
by
a
common
central
drive.
Differences
between
sympathetic
drives
to
arm
and
leg
muscles
have
been
demonstrated
during
mental
arithmetic
(Anderson,
Wallin
&
Mark,
1987)
and
post-
contraction
muscle
ischaemia
(Wallin,
Victor
&
Mark,
1989).
During
the
isometric
contraction
preceding
muscle
ischaemia
there
was
no
difference
between
sympathetic
outflows
to
resting
muscles
in
the
opposite
arm
and
the
leg
(Wallin
et
al.
1989),
but
no
such
recordings
have
been
made
from
nerves
innervating
contracting
muscles.
However,
there
is
indirect
evidence
that,
at
least
under
some
conditions,
there
may
be
increased
MSA
directed
to
exercising
muscle.
Strong
dynamic
contractions
of
thigh
muscles
to
50-100%
of
maximum
result
in
increased
noradrenaline
spillover
to
blood
from
the
working
muscle
(e.g.
Savard,
Strange,
Kiens,
Richter,
Christensen
&
Saltin,
1987),
suggesting
an
increase
in
sympathetically
mediated
vasoconstrictor
tone.
Similarly,
studies
in
intact
rats
and
following
acute
sympathectomy
suggest
that,
during
locomotion,
hindlimb
blood
flow
and
the
flow
to
individual
exercising
muscles
are
under
active
sympathetic
control
(Peterson,
Armstrong
&
Laughlin,
1988).
The
present
study
was
undertaken
to
quantify
the
degree
of
homogeneity
in
MSA
direct
to
muscles
of
both
legs
at
rest,
during
apnoea
and
during
isometric
voluntary
contractions
of
muscles
in
one
leg.
By
using
the
non-contracting
leg
as
a
control
it
was
possible
to
separate
regionally
specific
changes
in
MSA
from
generalized
changes.
METHODS
Thirteen
experiments
were
performed
on
twelve
normal
volunteers
(seven
male,
five
female,
aged
20-34
years)
with
their
informed
consent
and
with
the
approval
of
the
local
ethics
committee.
Successful
bilateral
recordings
of
sympathetic
activity
were
obtained
in
ten
experiments;
no
data
came
from
the
other
three
experiments.
The
subjects
lay
supine
on
a
comfortable
bed,
with
the
upper
body
supported
by
pillows,
the
left
leg
unrestrained
and
the
right
leg
secured
in
an
isometric
myograph.
At
the
start
of
each
experiment
maximal
dorsiflexion
and
plantar
flexion
forces
were
measured,
and
the
subject
was
trained
to
contract
tibialis
anterior,
extensor
digitorum
longus
or
triceps
surae
on
the
right
side.
The
electromyogram
(EMG)
of
appropriate
muscles
bilaterally
and
the
electrocardiogram
(ECG)
were
recorded
using
surface
electrodes.
Neural
recordings.
Recordings
of
MSA
were
made
bilaterally
using
tungsten
microelectrodes
introduced
manually
through
the
skin
into
the
right
and
left
peroneal
nerves
at
the
fibular
head
level.
Details
of
the
technique
and
evidence
for
the
sympathetic
nature
of
the
activity
have
been
220

SYMPATHETIC
ACTIVITY
DURING
CONTRACTION
described
elsewhere
(Sundl6f
&
Wallin,
1977;
Vallbo,
Hagbarth,
Torebj6rk
&
Wallin,
1979).
Fascicles
innervating
tibialis
anterior
were
sought
using
the
response
to
electrical
stimuli
delivered
through
the
microelectrode
as
a
guide.
In
nine
of
ten
experiments,
the
fascicle
from
which
MSA
was
recorded
on
the
right
side
innervated
tibialis
anterior;
in
the
tenth,
the
innervated
muscle
was
extensor
digitorum
longus.
When
the
tip
of
the
electrode
had
penetrated
the
desired
fascicle,
its
location
was
adjusted
carefully
under
auditory
control
to
bring
the
pulse-synchronous
bursts
of
MSA
into
focus.
On
request
subjects
remained
completely
relaxed,
held
their
breath
at
end-
expiration
('apnoea')
or
maintained
a
contraction
of
the
nominated
muscle
in
the
right
leg
for
up
to
6
min
at
a
specified
target
force
(5,
10,
20
or
30
%
of
maximal
voluntary
contraction,
MVC).
All
experimental
data
were
stored
on
tape
for
subsequent
analysis.
Sympathetic
activity
was
amplified
(x
10000-20000),
filtered
(bandpass
300
Hz-3
kHz)
and
stored
on
tape.
On
replay,
it
was
refiltered
prior
to
integration
using
a
RC
low-pass
filter
(time
constant
0-1
s).
This
produced
an
analog
signal
that
could
be
displayed
on
an
ink-jet
recorder
or
an
electrostatic
printer,
or
digitized
on
an
IBM-compatible
PC.
Sequences
were
rejected
when
contaminated
by
the
electromyogram
of
nearby
contracting
muscles.
Manual
quantification.
For
seven
subjects,
data
from
four
randomly
selected
2
min
sequences
when
the
subject
was
at
rest
and
one
when
apnoeic
were
replayed
on
an
ink-jet
recorder,
with
amplification
adjusted
such
that
the
sizes
of
corresponding
bursts
in
the
two
recordings
were
similar.
The
amplitudes
of
each
burst
of
sympathetic
activity
in
the
two
recordings
were
measured
for
the
five
sequences
for
the
seven
subjects,
expressing
each
burst
as
a
percentage
of
the
largest
burst
in
that
sequence.
The
amplitude
of
a
burst
in
the
right
peroneal
recording
was
then
plotted
against
the
amplitude
of
the
corresponding
burst
in
the
left
peroneal
recording
for
each
sequence.
Regression
analysis
was
performed
on
these
data,
and
the
correlation
coefficient
taken
as
an
index
of
the
degree
of
homology
of
the
two
recordings
(see
Wallin
et
al.
1989).
Computer
analysis.
The
integrated
neurograms
of
the
right
and
left
peroneal
nerve
sympathetic
activity
were
digitized
in
sequences
of
2
min
duration
using
a
sampling
rate
of
0-1
kHz.
Auto-
and
cross-correlograms
were
derived
from
the
digitized
data,
and
power-spectral
analysis
then
performed
on
these
correlograms
(see
Fig.
2).
The
cardiac
period
was
identified
from
the
ECG
recording
and
confirmed
in
the
auto-correlograms.
The
power
in
each
recording
was
measured
at
the
cardiac
period.
From
the
cross-correlated
data
the
coherence
between
the
two
recordings
and
the
gain
of
the
relationship
between
the
two
were
measured
at
the
cardiac
period
(assuming
that
the
left
side
was
the
input
to
and
the
right
the
output
from
the
cross-correlogram).
Gain
was
computed
as
cross-spectral
power
divided
by
the
input
(left
side)
spectral
power.
Coherence
was
calculated
as
the
square
of
the
cross-spectral
power
divided
by
the
product
of
the
input
and
output
powers.
Because
these
measurements
were
made
at
the
cardiac
period,
only
noise
at
the
cardiac
period
could
have
affected
the
measurements.
This
assumption
was
confirmed
in
control
studies
in
which
a
neurogram
was
correlated
with
itself
after
noise
was
added
to
one
input.
The
added
noise
was
bandpass-filtered
white
noise
(300
Hz-3
kHz).
In
different
control
runs
this
added
signal
was
held
constant,
varied
randomly
or
varied
in
time with
the
ECG.
Only
the
latter
decreased
the
coherence
between
the
two
signals
by
>
5
%.
This
finding
implies
that
increases
in
non-sympathetic
neural
traffic
during
a
contraction
(or
undetected
contamination
of
the
neural
recording
by
EMG
activity
would
affect
the
measurements
only
if
they
possessed
a
cardiac
periodicity.
Although
some
non-sympathetic
neural
activity
does
have
a
cardiac
periodicity
(McKeon
&
Burke,
1981),
such
contamination
would
increase
the
power
in
the
relevant
recording:
in
the
studies
reported
here,
contraction
was
associated
with
a
decrease
in
power
at
the
cardiac
frequency.
In
similar
controls,
changing
the
amplification
of
one
input,
slowly
or
abruptly,
produced
changes
in
coherence
of
<
5
%
provided
that
the
gain
change
was
not
locked
to
the
cardiac
period.
This
finding
implies
that
coherence
would
change
minimally
with
minor
disturbances
to
electrode
position,
were
this
to
occur
on
the
right
side
during
contraction
sequences.
Such
changes
would,
however;
appear
as
a
change
in
gain
between
the
two
recordings,
much
as
is
shown
in
Fig.
3.
RESULTS
Satisfactory
bilateral
recordings
of
sympathetic
efferent
activity
to
pretibial
muscles
were
obtained
in
ten
experiments
on
nine
subjects.
In
all
recordings
there
was,
at
rest,
a
striking
similarity
in
the
pattern
and
amplitude
of
the
pulse-
synchronous
bursts
of
sympathetic
activity
destined
for
the
muscles
of
opposite
legs
221

B.
G.
WALLIN,
D.
BURKE
AND
S.
C.
GANDEVIA
Rest
5s
Apnoea
Fig.
1.
Bilateral
recordings
of
muscle
nerve
sympathetic
activity
from
the
right
and
left
peroneal
nerves
showing
the
similarity
of
the
integrated
neurograms.
In
each
panel
the
upper
trace
is
the
neurogram
from
the
right
leg,
the
second
trace
the
neurogram
from
the
left
leg,
and
the
third
trace
ECG.
Bursts
which
appear
only
on
one
side
are
indicated
by
asterisks.
In
the
lower
panel,
the
subject
was
apnoeic
for
the
duration
indicated
by
the
horizontal
bar.
A
8
100
10
a)~
~
~~~ne
0
oo
O
C
D
100-
100-
Apnoea
OR~~~~~~~~~~~~~~~~~~~a
g0
0
a
T
ApeApnoea
.50
0L50
o
0
Rest0
est
0
1-5
3
0
0
1-5
3.0
Frequency
(Hz)
Frequency
(Hz)
Fig.
2.
Computer
analysis
of
2
min
recordings,
samples
of
which
are
illustrated
in
Fig.
1.
Apnoea
resulted
in
a
marked
increase
in
power
bilaterally
but
only
slight
changes
in
gain
and
coherence.
During
apnoea
the
heart
rate
increased
from
092
to
1-02
Hz.
222

SYMPATHETIC
ACTIVITY
DURING
CONTRACTION
223
(Fig.
1,
upper
panel).
There
were,
however,
small
bursts
that
could
be
identified
only
on
one
side
(indicated
by
asterisks
in
Fig.
1),
and
often
the
amplitude
of
a
single
burst
did
not
have
the
same
relationship
to
those
of
other
bursts
in
the
corresponding
sequence
on
the
other
side.
For
the
subject
illustrated
in
Fig.
1,
the
correlation
New
site
I'
!
,
o.
.
,
.
Nt
''
I
t
I
.
I
t
I
.
'
1
/
.
1:
|
__.
_
TA
TA
*10%'
20%
if
\
21
.d
I
\,
C)
1'N
!.
'
.
.
:
!:
TA
Tri
EDL.
30%
10%
1
o%
,/
'
'a
Fig.
3.
The
effect
of
different
manoeuvres
on
coherence
and
gain
plotted
sequentially
throughout
an
experiment.
*
represent
values
obtained
at
rest,
*
represent
values
obtained
when
apnoea
was
performed
by
an
otherwise
resting
subject,
0
represent
values
obtained
during
contraction,
and
[1
represent
values
obtained
when
the
subject
was
apnoeic
during
the
contraction.
The
resting
values
for
coherence
changed
little
during
the
experiment,
as
indicated
by
the
error
bars
on
either
side
of
the
plot
(±
1
S.D.).
Coherence
changed
little
with
contractions
of
right
tibialis
anterior
(TA),
right
triceps
surae
(Tri)
or
right
extensor
digitorum
longus
(EDL)
at
10%.
There
were
significant
decreases
in
coherence
with
contractions
of
right
TA
at
20
and
30%
MVC.
In
the
initial
part
of
the
experiment
there
is
a
steady
increase
in
gain,
due
to
progressive
dislodgement
of
the
microelectrode
on
the
left
side.
Despite
this,
coherence
values
at
rest
remained
quite
reproducible.
At
the
dotted
line
the
experiment
was
interrupted
until
a
stable
recording
was
obtained
bilaterally.
coefficient
between
corresponding
bursts
on
the
two
sides
was
083
at
rest
and
0-92
during
apnoea.
Figure
2
illustrates
the
power
spectral
analyses
for
these
two
recordings.
The
computed
coherences
for
these
two
recordings
were
92-4
and
96-4
%,
at
the
cardiac
frequencies
092
Hz
and
1P02
Hz,
respectively.
2.0
r
15
1-
c
U7
10
1
05
F
O
L
100
r
I
80
60
I-
0)
O
c
0)
0)
0
U
40
_
20
F
0
I

B.
G.
WALLIN,
D.
BURKE
AND
S.
C.
GANDEVIA
Homogeneity
of
the
sympathetic
drive
to
muscles
of
different
limbs
at
rest
In
each
experiment
the
coherence
between
the
two
recordings
was
computed
for
the
subject
at
rest,
over
five
to
fifteen
2
min
sequences
(average
10-2
sequences/experiment).
The
degree
of
coherence
between
the
two
recordings
varied
1-0r
08
F
061-
._
0-41
02
I
0
100
.XII
TA
.
10%,
,19.
S
I
z
1:
./
90
I
a,
1-.
a,
a,
0
u
80
F
701-
New
site
*
*
*
|
TA
TA
Tri
*
EDL
t
5%
20%
10% 10%
s
r
o
I!
\
\
I
,
,
60
F
50
Fig.
4.
The
evolution
of
coherence
and
gain
values
throughout
an
experiment
in
a
second
subject.
The
symbols
are
as
in
Fig.
3.
The
reproducibility
of
the
coherence
when
at
rest
is
indicated
by
the
error
bars
on
either
side
of
the
coherence
plot
(±1
S.D.).
Coherence
and
gain
decreased
in
the
three
contractions
of
right
TA
but
changed
little
in
the
contractions
of
right
triceps
surae
and
EDL.
The
recording
site
on
the
right
side
was
lost
abruptly
at
the
vertical
arrow,
so
that
the
subsequent
recordings
had
a
different
gain.
However,
the
coherence
values
at
rest
were
quite
reproducible.
throughout
an
experiment.
Some
of
this
variability
appeared
to
be
related
to
a
preceding
manoeuvre,
particularly
apnoea,
following
which
return
to
a
truly
basal
state
took
several
minutes
(Figs
3
and
4).
In
the
ten
experiments
the
average
coherence
of
all
sequences
at
rest
was
65A4-92-2
%
(mean
82-1
%,
S.D.
7
9
%).
In
some
subjects
the
coherence
values
for
all
rest
sequences
in
the
experiment
had
S.D.S
of
2-4
%
despite
many
manoeuvres.
The
experiments
with
the
highest
variability
had
S.D.S
of
9
5
and
12-8%,
respectively.
224

SYMPATHETIC
ACTIVITY
DURING
CONTRACTION
The
difference
in
coherence
between
consecutive
rest
sequences
with
no
intervening
apnoea,
contraction
or
change
in
recording
site
was
determined
to
quantify
the
reproducibility
of
coherence
measurements
in
the
absence
of
these
manoeuvres.
The
mean
difference
in
coherence
in
forty-nine
consecutive
pairs
of
measurements
was
100-
50
.
0~~~~~~~~~4
-D
0
0
50-
0-25
0-5
0-75
10
Correlation
coefficient
Fig.
5.
Relationship
between
coherence
and
correlation
coefficient
for
seven
subjects.
The
values
for
sequences
when
the
subject
was
at
rest
are
indicated
by
*.
The
values
for
the
same
subjects
when
apnoeic
are
shown
by
0.
The
line
represents
the
regression
line
for
all
data
(r
=
0-60,
P
<
0-01).
4-2
%
(S.D.
4-3%).
In
nine
of
the
ten
experiments,
the
mean
differences
were
0
1-6
5
%;
in
the
tenth
experiment,
one
of
only
three
values
was
25-2
%.
Of
the
forty-
nine
consecutive
differences,
all
but
two
were
<
10%.
For
seven
subjects,
the
amplitudes
of
corresponding
bursts
were
measured
manually
for
four
sequences
at
rest,
and
linear
regression
was
performed
on
these
measurements
to
provide
correlation
coefficients
as
a
measure
of
the
similarity
of
the
activity
on
the
two
sides.
The
mean
correlation
coefficient
of
the
four
rest
sequences
for
the
seven
subjects
was
0-72
(S.D.
0410)
indicating
that,
in
these
sequences,
52
%
of
the
variance
in
the
two
recordings
could
be
attributed
to
common
drives.
For
these
same
twenty-eight
sequences,
the
mean
coherence
was
85-6
%
(S.D.
6-7
%).
The
sequences
analysed
for
each
subject
were
usually
taken
from
different
stages
of
the
experiment.
However,
there
were
seven
pairs
of
consecutive
rest
sequences,
and
the
mean
difference
in
correlation
coefficient
for
consecutive
sequences
was
0-08.
The
mean
difference
in
coherence
for
the
same
seven
pairs
of
analyses
was
5-7
%.
When
the
data
for
the
seven
subjects
were
pooled,
there
was
a
significant
linear
relationship
between
coherence
and
correlation
coefficient
for
the
same
sequences
at
rest
(Fig.
5,
*;
r
=
0-55,
P
<
0-01).
This
relationship
was
improved
when
the
larger
bursts
that
occur
during
apnoea
(see
later)
were
included
(Fig.
5,
0;
r
=
0-60,
P
<
00
1).
Including
the
data
obtained
during
apnoea
did
not
alter
the
slope
of
the
relationship.
A
positive
correlation
between
coherence
and
correlation
coefficient
was
225
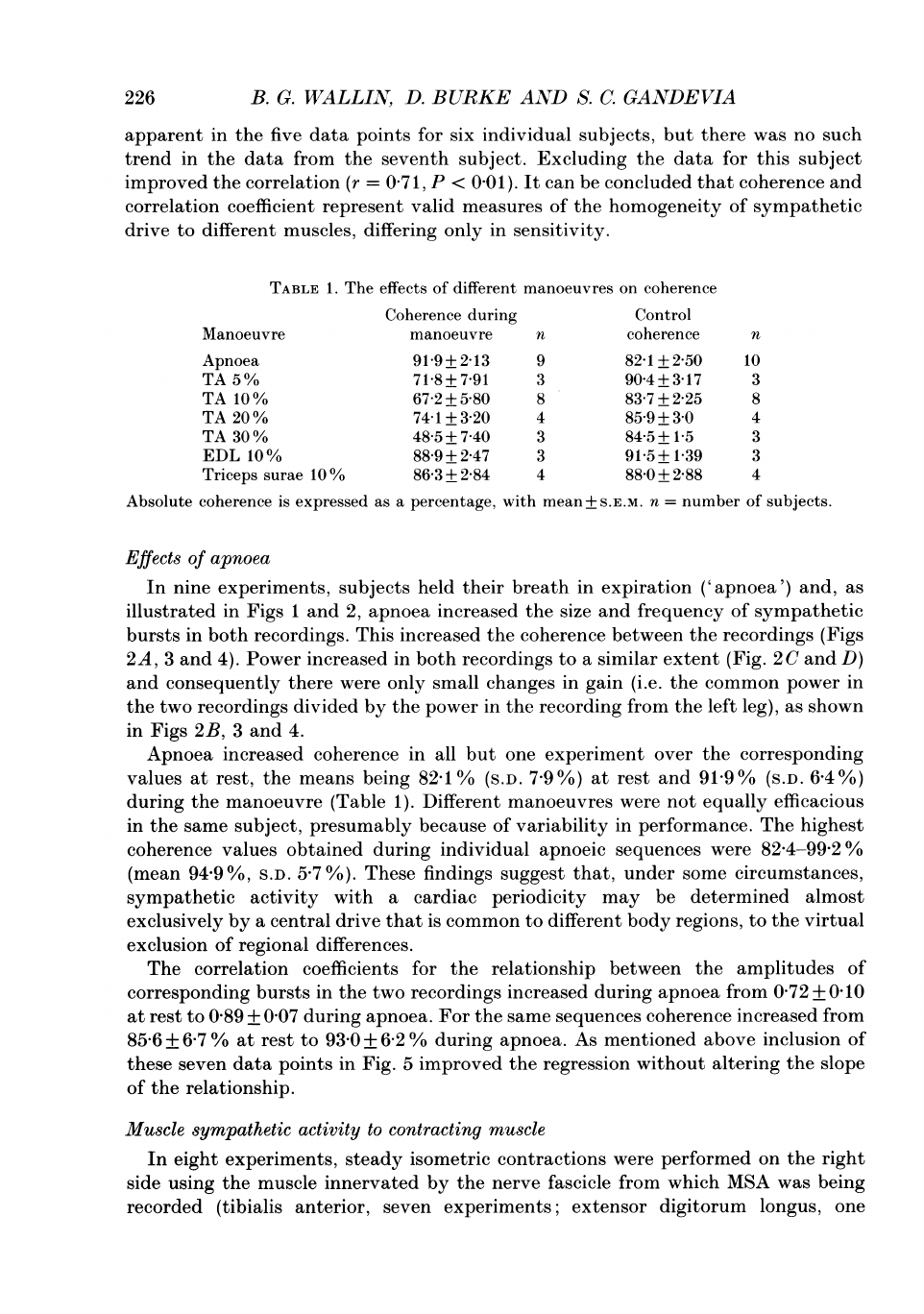
226
B.
G.
WALLIN,
D.
BURKE
AND
S.
C.
GANDEVIA
apparent
in
the
five
data
points
for
six
individual
subjects,
but
there
was
no
such
trend
in
the
data
from
the
seventh
subject.
Excluding
the
data
for
this
subject
improved
the
correlation
(r
=
07
1,
P
<
00
1).
It
can
be
concluded
that
coherence
and
correlation
coefficient
represent
valid
measures
of
the
homogeneity
of
sympathetic
drive
to
different
muscles,
differing
only
in
sensitivity.
TABLE
1.
The
effects
of
different
manoeuvres
on
coherence
Coherence
during
Control
Manoeuvre
manoeuvre
n
coherence
n
Apnoea
919+213
9
821+250
10
TA
5%
71
8+791
3
904+3
17
3
TA
10%
672+580
8
837+225
8
TA
20%
74-1+3-20
4
85-9+3-0
4
TA
30%
48-5+740
3
84-5+1-5
3
EDL
10%
88-9+2-47
3
91-5+1-39
3
Triceps
surae
10%
86-3
+
2-84
4
88-0
+
2-88
4
Absolute
coherence
is
expressed
as
a
percentage,
with
mean+
S.E.M.
n
=
number
of
subjects.
Effects
of
apnoea
In
nine
experiments,
subjects
held
their
breath
in
expiration
('apnoea')
and,
as
illustrated
in
Figs
1
and
2,
apnoea
increased
the
size
and
frequency
of
sympathetic
bursts
in
both
recordings.
This
increased
the
coherence
between
the
recordings
(Figs
2A,
3
and
4).
Power
increased
in
both
recordings
to
a
similar
extent
(Fig.
2
C
and
D)
and
consequently
there
were
only
small
changes
in
gain
(i.e.
the
common
power
in
the
two
recordings
divided
by
the
power
in
the
recording
from
the
left
leg),
as
shown
in
Figs
2B,
3
and
4.
Apnoea
increased
coherence
in
all
but
one
experiment
over
the
corresponding
values
at
rest,
the
means
being
82-1
%
(S.D.
7-9%)
at
rest
and
91
9°%
(S.D.
6-4
%)
during
the
manoeuvre
(Table
1).
Different
manoeuvres
were
not
equally
efficacious
in
the
same
subject,
presumably
because
of
variability
in
performance.
The
highest
coherence
values
obtained
during
individual
apnoeic
sequences
were
82-4-99-2
%
(mean
94-9
%,
S.D.
5.7
%).
These
findings
suggest
that,
under
some
circumstances,
sympathetic
activity
with
a
cardiac
periodicity
may
be
determined
almost
exclusively
by
a
central
drive
that
is
common
to
different
body
regions,
to
the
virtual
exclusion
of
regional
differences.
The
correlation
coefficients
for
the
relationship
between
the
amplitudes
of
corresponding
bursts
in
the
two
recordings
increased
during
apnoea
from
072
+
O-10
at
rest
to
089
+
007
during
apnoea.
For
the
same
sequences
coherence
increased
from
85-6
+
6-7
%
at
rest
to
93
0
+
6-2
%
during
apnoea.
As
mentioned
above
inclusion
of
these
seven
data
points
in
Fig.
5
improved
the
regression
without
altering
the
slope
of
the
relationship.
Muscle
sympathetic
activity
to
contracting
muscle
In
eight
experiments,
steady
isometric
contractions
were
performed
on
the
right
side
using
the
muscle
innervated
by
the
nerve
fascicle
from
which
MSA
was
being
recorded
(tibialis
anterior,
seven
experiments;
extensor
digitorum
longus,
one

SYMPATHETIC
ACTIVITY
DURING
CONTRACTION
227
experiment).
Simultaneous
recordings
of
sympathetic
activity
were
obtained
from
the
left
(control)
leg
and
from
the
contracting
right
leg.
The
voluntary
contractions
increased
the
background
neural
noise
generated
by
muscle
afferents
and
non-
sympathetic
efferents
in
the
nerve
fascicle
innervating
the
contracting
muscle.
This
150
-
*
Mean
-M
No.
1
_
EI
No.
2
0
0
0
80_
0
410
C
~0
0
Coherence
Gain
Power
left
Power
right
leg
leg
Fig.
6.
The
changes
in
coherence,
gain
and
power
for
three
subjects
during
contractions
of
right
TA
at
5%
MVC.
Data
are
normalized
as
percentages
of
the
pre-contraction
control
values.
For
each
data
set,
the
filled
bar
represents
the
mean
of
the
data
for
the
three
subjects.
Coherence
decreased
in
all
three
subjects,
as
did
gain.
The
power
in
the
recording
from
the
contracting
(right)
leg
decreased
in
all
three
subjects
but,
on
average,
there
was
no
change
in
the
power
from
the
non-contracting
(left)
leg.
increase
in
noise
invalidated
manual
measurements
of
burst
amplitude
from
that
side
but
did
not
alter
the
spectral
analysis
significantly
because
it
did
not
contribute
power
at
the
cardiac
frequency
(see
Methods).
Furthermore,
measurements
of
gain
are
dependent
only
on
the
cross-correlated
(common)
power
in
the
recording
from
that
leg.
Coherence
decreased
in
all
contractions,
no
matter
what
the
contraction
strength
(Table
1).
In
five
of
six
experiments
in
which
contractions
of
different
strength
were
tested,
the
stronger
the
contraction
the
greater
was
the
decrease
in
coherence
(Fig.
3A).
However,
this
relationship
disappeared
in
pooled
data
(see
below),
because
different
individuals
were
tested
with
different
contraction
levels
and
there
was
marked
interindividual
variability
in
the
extent
of
the
decrease.
With
contractions
of
different
strength
(5,
10,
20
and
30%
MVC),
the
average
decrease
in
coherence
exceeded
10%
at
each
level
(Figs
6-8).
The
decrease
in
coherence
was
accompanied
by
a
decrease
in
power
in
the
recording
from
the
contracting
leg
at
each
contraction
level.
This
change
in
power
was
not
statistically
significant
at
any
of
the
levels,
but
when
the
data
were
pooled
for
all
contraction
levels
in
all
subjects,
it
was
statistically
significant,
whether
the
power
during

228
B.
G.
WALLIN,
D.
BURKE
AND
S.
C.
GANDEVIA
contraction
was
compared
with
that
in
the
preceding
rest
period
(mean
decrease
10-0
%;
P
=
0-0079;
two-tailed
paired
t
test)
or
with
the
mean
of
the
power
values
for
the
preceding
and
subsequent
rest
periods
(mean
decrease
13-8
%
P
=
0-00
13;
two-
tailed
paired
t
test).
100
-v
01)
0)
c
a)
_C
0
u
50
20
::
.
.n
A:
.
.:>:
>:
.....
>
-:
-:.
::>
Is
--
n
An.+.
so.
Is
sesns'nsn
:.:.:.:.:.:.:.:.:.-.:.:.-
->:-'-F::
F:
:-F:
:-
:S
>:
:->:
e:::>::
::2:-::2:
.:
::.:-::::.:::::.-e.
:.-
:.:.:.:.:.:.:.:.:.:
:::
.;;:.:
:.:.
:.:
>.-:
>:-F>:-'-:-'-'-:
:*:
:-X
:-:-
-.
;:-
>::
>:'
>:
->:
>:
IS
nt..
In
FS-
::S::::;-:::S::::S:
::
F.:
':F,:::::
S:
.............
.............
......
..............
............
...............
........
..........
.............
..
..............
..............
........
:E
lectro
chemi
cal
0
1
00
01
cm
...........
..
..............
........
....
...
......
....
..
... ... ...
..
100
CD
C
...........
Co
............
cm
-T-
......
...............
.......
...........
...
.............
.............:
...........
.............
............. ............. .............
0
C
0
C:
M
0
M
M
0
CI4
CD
U
(1)
M
Q)
-
CY)
0
0
0
M
lo-o
C
C
x
C
co
CD
CL
0
CL
cc
cc
<
5
<
T
Cr
Fig.
7.
Data
for
contractions
of
right
TA
at
IO
%
MVC.
Each
column
represents
the
mean
for
eight
subjects
(±
I
S.E.M.).
There
was
no
significant
contraction-induced
change
in
power
for
the
non-
contracting
leg
when
the
power
value
during
the
contraction
was
compared
to
that
in
the
preceding
rest
period
(mean
decrease
5-7
%;
P
=
0-2366;
two-tailed
paired
t
-T

SYMPATHETIC
ACTIVITY
DURING
CONTRACTION
229
test).
When
compared
to
the
mean
of
the
power
values
for
the
preceding
and
following
rest
periods,
there
was
a
significant
decrease
in
power,
though
to
a
lesser
extent
than
on
the
contracting
side
(mean
decrease
7-2
%;
P
=
0-0345;
two-tailed
paired
t
test).
As
a
result
the
gain
between
the
two
sides
generally
decreased
(fifteen
120
U,
0
00
Cu
80
0
80
Cy)
C
~0
Co60-
co
CD
Co
40
40
~0
Coherence
Gain
Power
left
Power
right
leg
leg
Fig.
8.
Pooled
data
for
contractions
of
right
TA
at
20
and
30%
MVC,
expressed
as
percentages
of
the
pre-contraction
control
values
(n
=
4
at
20%;
n
=
3
at
30%).
Each
column
represents
the
mean
(±
1
S.E.M.).
There
are
decreases
in
coherence,
gain
and
the
power
for
the
contracting
(right)
leg,
but
no
change
in
the
power
for
the
non-contracting
(left)
leg.
of
eighteen
trials).
This
change
could
be
seen
in
the
mean
data
for
each
contraction
level,
except
at
10
%
MVC
(Figs
6-8),
and
was
significant
when
the
data
for
all
trials
were
pooled
(P
=
0-0085).
In
determining
the
effects
of
contraction
on
power,
the
preferred
comparison
was
with
the
power
in
the
pre-contraction
rest
period
rather
than
with
the
mean
power
in
the
pre-
and
post-contraction
rest
periods.
The
usual
experimental
protocol
involved
performance
of
apnoea
at
the
end
of
a
contraction
and
again
immediately
after
relaxation,
prior
to
the
post-contraction
rest
period
(see
Figs
3
and
4).
In
some
sequences,
the
increase
in
power
due
to
apnoea
subsided
slowly,
continuing
into
the
post-contraction
rest
period.
Similarly,
apnoea
prior
to
contraction
(see
Fig.
6)
may
have
falsely
elevated
the
mean
power
during
the
contraction
in
some
sequences,
but
this
would
have
minimized
the
contraction-induced
change,
not
enhanced
it.
Apnoea
during
contraction
Sustained
apnoea
during
a
contraction
of
the
homonymous
muscle
restored
coherence,
often
above
the
control
values
at
rest
but
below
values
achieved
with
apnoea
when
not
contracting
(Figs
3,
4
and
7).
The
mean
increases
in
coherence
were
18-7,
16-5
and
15-7
%
with
contraction
levels
of
10,
20
and
30%
MVC
respectively.
However,
when
the
apnoea
was
repeated
on
cessation
of
the
contraction,
coherence
increased
by
a
further
6-3,
3-3
and
19-3
%,
respectively.
These
changes
occurred
in
all
trials.
During
contraction,
apnoea
increased
the
power
in
recordings
from
both
the

B.
G.
WALLIN,
D.
BURKE
AND
S.
C.
GANDEVIA
contracting
and
the
non-contracting
legs,
generally
in
parallel
(Fig.
7),
but
it
increased
the
gain
in
ten of
the
fifteen
trials
due
to
a
slightly
greater
effect
on
the
contracting
leg.
As
a
result
apnoea
reduced
the
asymmetry
in
sympathetic
outflow
produced
by
contraction.
Contraction
of
other
muscles
The
effects
of
contracting
a
synergist
(extensor
digitorum
longus,
at
10
%
MVC;
three
experiments)
or
an
antagonist
(triceps
surae,
at
10
%
MVC;
four
experiments)
were
tested
(Figs
3
and
4;
Table
1).
With
contraction
of
the
synergist,
there
was
little
change
in
coherence
(on
average
a
decrease
of
2-6
%),
even
though
one
subject
found
it
difficult
to
prevent
contraction
of
tibialis
anterior
when
holding
a
steady
contraction
of
extensor
digitorum
longus.
With
contractions
of
triceps
surae,
there
was
on
average
a
decrease
in
coherence
of
1-7
%.
With
neither
muscle
were
these
changes
significant;
both
changes
fall
well
within
the
mean
reproducibility
of
coherence
measures
for
consecutive
sequences
(4-2
%,
see
earlier).
Apnoea
when
contracting
a
synergist
or
antagonist
had
similar
effects
as at
rest.
DISCUSSION
The
present
results
have
quantified
the
similarity
between
the
sympathetic
efferent
drives
to
homologous
muscles
in
different
limbs
at
rest,
and
demonstrated
even
greater
similarity
when
subjects
hold
their
breath
in
expiration.
There
is
a
significant
decrease
in
this
similarity
when
one
of
the
homologous
muscles
contracts,
and
this
is
largely
due
to
a
decrease
in
sympathetic
drive
to
the
contracting
muscle.
Contraction
of
a
synergist
or
antagonist
at
10
%
maximal
force
has
little
effect
on
the
symmetry
of
sympathetic
outflow.
MSA
has
a
prominent
cardiac
rhythmicity,
and
power,
gain
and
coherence
were
therefore
measured
at
the
cardiac
period.
The
analysis
would
not
have
measured
all
sympathetic
activity
because
power
at
multiples
of
the
cardiac
period
was
ignored.
However,
the
effects
of
this
omission
were
small:
the
peak
at
the
cardiac
period
dominated
the
power
spectrum
(see
Fig.
2),
and
inspection
of
the
plots
indicated
that
the
peaks
at
the
cardiac
period
and
at
twice
the
period
behaved
in
much
the
same
way
during
contractions.
During
apnoea
more
heart
beats
were
associated
with
sympathetic
bursts
than
in
the
control
situation,
and
this
change
may
have
accentuated
the
increase
in
power
at
the
cardiac
period.
Homogeneity
of
sympathetic
drives
Total
sympathetic
outflow
presumably
depends
on
a
balance
between
drives
that
are
common
to
all
muscle
groups,
and
regionally
specific
drives
of
central
or
peripheral
(reflex)
origin.
In
subjects
at
rest
there
is
probably
no
specific
stimulus
for
regional
variations
in
muscle
blood
flow
and,
accordingly,
the
coherence
between
sympathetic
drives
to
muscles
of
different
limbs
was
high
in
relaxed
subjects.
During
apnoea
the
sympathetic
outflow
to
muscle
increases
but
again
there
is
no
reason
why
this
drive
should
be
distributed
unevenly
to
the
two
legs.
Coherence
decreased
when
one
of
the
innervated
muscles
was
contracted.
This
implies
that
the
common
drive
had
become
less
dominant
and,
by
inference,
that
230

SYMPATHETIC
ACTIVITY
DU
RING
CONTRACTION1
regional
drives
were
more
important.
However,
this
inference
is
valid
only
if
a
change
in
noise
can
be
excluded
as
the
cause
for
the
decreased
coherence.
Inevitably
contraction
is
associated
with
increased
afferent
and
efferent
traffic
in
the
nerve
fascicle
innervating
the
contracting
muscle.
but
this
is
unlikely
to
have
affected
the
measurements
for
two
reasons.
First,
an
increase
in
noise
would
be
expected
to
increase
the
power
in
the
recording
from
the
contracting
leg.
not
decrease
it
as
was
consistently
found.
Secondly,
the
measurements
of
power,
gain
and
coherence
were
made
at
the
cardiac
period
because
MSA
occurs
in
pulse-synchronous
bursts.
Control
studies
indicate
that
only
changes
in
noise
that
occur
with
a
cardiac
periodicity
affect
coherence
(see
Methods).
Thus,
regional
factors,
central
or
peripheral,
assume
greater
importance
in
determining
sympathetic
outflow
when
subjects
contract a
muscle
than
when
they
are
at
rest.
In
anaesthetized
and
decerebrate
cats
with
all
baroreceptors
denervated.
Koesis.
Gebber,
Barman
&
Kenney
(1990)
determined
the
coherence
between
different
sympathetic
nerve
pairs
using
a
similar
analysis
to
that
used
here.
There
was
correlated
activity
in
the
2-6
Hz
range
with
different
phase
relationships
that
could
be
altered
experimentally
(e.g.
by
altering
ventilation
rate).
These
analyses
indicate
that
the
relationship
between
different
sympathetic
outflows
is
not
immutable,
a
conclusion
supported
by
the
present
findings.
Their
coherence
values
were
generally
lower
than
in
the
present
study,
some
50-70
%,
and
this
raises
the
question
whether
the
present
analysis,
restricted
to
power
at
the
cardiac
period,
might
have
resulted
in
high
coherence
values
that
exaggerate
the
degree
of
similarity
between
the
correlated
recordings.
In
this
context,
it
is
notable
that
the
correlation
coefficient
of
the
relationship
between
the
amplitudes
of
equivalent
sympathetic
bursts
suggests
that,
at
rest,
52
%
of
the
variance
of
MSA
in
the
two
recordings
was
due
to
a
common
drive,
less
than
suggested
by
the
coherence
value.
On
the
other
hand,
the
baroreflex
is
responsible
for
the
pulse-synchronous
bursts
seen
in
human
MSA
(Wallin
&
Fagius,
1988),
and
this
would
impose
a
high
degree
of
similarity
for
nerves
innervating
muscle
throughout
the
body.
Vasoconstrictor
or
vasodilator
activity
There
is
strong
evidence
that
the
pulse-synchronous
multiunit
bursts
of
MSA
are
dominated
by
vasoconstrictor
impulses
which
are
influenced
by
arterial
baroreflexes
(Vallbo
et
al.
1979;
Vissing,
Scherrer
&
Victor,
1989).
Vasodilator
efferents
are
present
in
the
skin
of
the
human
forearm
and
lower
leg
(Grant
&
Holling,
1938)
and
foot
(Lundberg,
Norgren,
Ribbe,
Rosen.
Steen,
Thorne
&
Wallin,
1989)
and,
although
the
question
is
not
settled,
some
observations
suggest
that
human
muscle
receives
an
active
vasodilator
innervation
(Blair,
Glover,
Greenfield
&
Roddie,
1959).
However,
muscle
vasodilator
neurones
in
the
cat
are
neither
spontaneously
active
at
rest
nor
influenced
by
arterial
baroreceptors
(Horeyseck,
Jhnig,
Kirchner
&
Thhmer,
1972,
1976).
Thus,
even
if
a
putative
vasodilator
innervation
of
human
muscle
were
important,
the
present
analyses
have
probably
avoided
such
activity
by
focusing
on
pulse-synchronous
activity
and
activity
in
correlograms
with
a
cardiac
periodicity.
231

B.
G.
WALLIN,
D.
BURKE
AND
S.
C.
GANDEVIA
Changes
in
sympathetic
activity
associated
with
voluntary
contraction
The
findings
of
decreased
coherence
between
the
recordings
from
the
two
legs
and
decreased
power
in
the
recording
from
the
contracting
leg
suggest
that
contraction
of
a
lower
limb
muscle
at
5-30
%
MVC
induced
a
local
reduction
of
sympathetic,
presumably
vasoconstrictor,
drive
to
that
muscle.
The
specificity
of
this
alteration
is
illustrated
by
the
fact
that
no
such
change
was
found
when
the
contraction
involved
not
the
innervated
muscle
but
a
neighbour
in
the
same
leg,
be
it
a
synergist
or
antagonist.
A
decrease
in
MSA
would
certainly
add
to
metabolic
factors
tending
to
produce
vasodilatation
in
the
contracting
muscle.
Whether
the
underlying
mechanism
for
the
MSA
reduction
is
a
descending
supraspinal
influence
or
an
inhibitory
spinal
reflex
is
unclear.
There
has
been
no
previous
study
of
changes
in
MSA
to
contracting
muscles,
but
one
study
of
noradrenaline
spillover
led
to
the
opposite
conclusions
(Savard
et
al.
1987).
However,
that
study
involved
dynamic
(rather
than
static)
contractions
of
a
large
muscle
mass
performed
at
50-100
%
of
maximal
power
for
10-20
min.
Compared
to
our
weak,
fairly
short-lasting
contractions
such
efforts
should
be
expected
to
produce
a
much
greater
cardiovascular
load
and
therefore
it
would
be
reasonable
to
expect
a
difference
in
vasoregulatory
neural
outflow.
A
number
of
studies
have
addressed
changes
in
MSA
to
lower
limb
muscles
during
sustained
contractions
of
muscles
of
the
forearm
or
jaw
(Delius,
Hagbarth,
Hongell
&
Wallin,
1972;
Mark,
Victor,
Nerhed
&
Wallin,
1985;
Seals,
1989;
Elam,
Johansson
&
Wallin,
1991).
The
common
finding
was
an
increase
in
MSA,
particularly
after
contractions
lasting
more
than
1
min.
The
degree
of
MSA
increase
is
load
and
time
dependent
(Saito,
Mano,
Abe
&
Iwase,
1986;
Seals
&
Enoka,
1989)
and
has
been
found
to
correlate
to
a
decrease
of
intramuscular
pH
in
the
contracting
muscles
(Victor,
Bertocci,
Pryor
&
Nunnally,
1988).
In
contrast,
recent
studies
suggest
that
static
leg
exercise
does
not
increase
MSA
to
the
non-contracting
leg
(Ray,
Rea,
Clary
&
Mark,
1990,
1991).
This
finding
is
consistent
with
the
present
results.
This
work
was
supported
by
the
National
Health
and
Medical
Research
Council
of
Australia,
the
Ramaciotti
Foundations
and
the
Swedish
Medical
Research
Council
(grant
B91-04X-03546-20A).
We
would
like
to
thank
G.
Macefield,
J.
P.
Hales
and
R.
B.
Gorman
for
programming
and
for
assistance
with
experiments
and
analysis.
REFERENCES
ANDERSON,
E.
A.
WALLIN,
B.
(".
&
MARK,
A.
L.
(1987).
Dissociation
of
sympathetic
nerve
activity
in
arm
and
leg
muscle
during
mental
stress.
Hypertension
6,
suppl.
III,
114-119.
BLAIR,
D.
A..
GLOVER,
W.
E.,
GREENFIELD,
A.
D.
M.
&
RoDDIE,
I.
C.
(1959).
Excitation
of
cholinergic
vasodilator
nerves
to
human
skeletal
muscles
during
emotional
stress.
Journal
of
Physiology
148.
633-647.
DELIUS.
W.,
HAGBARTH.
K.-E.,
HONGELL,
A.
&
WALLIN,
B.
G.
(1972).
Manoeuvres
affecting
sympathetic
outflow
in
human
muscle
nerves.
Acta
Physiologica
Scandinavica
84,
82-94.
ELAM,
M.,
JOHANSSON.
G.
&
WALLIN,
B.
G.
(1992).
Do
patients
with
primary
fibromyalgia
have
an
altered
muscle
sympathetic
nerve
activity?
Pain
48,
371-375.
GRANT,
R.
T.
&
HOLLING,
H.
E.
(1938).
Further
observations
on
the
vascular
responses
of
the
human
limb
to
body
warming:
evidence
for
sympathetic
vasodilator
nerves
in
the
normal
subject.
Clinical
Science
3,
273-285.
232

SYMPATHETIC
ACTIVITY
DURING
CONTRACTION
HOREYSECK,
G.,
JANIG,
W.,
KIRCHNER,
F.
&
THXMER,
F.
&
THXMER,
V.
(1972).
Activation
of
muscle
vasodilator
neurons
by
hypothalamic
stimulation.
Brain
Research
48,
394-396.
HOREYSECK,
G.,
JANIG,
W.,
KIRCHNER,
F.
&
THXMER,
V.
(1976).
Activation
and
inhibition
of
muscle
and
cutaneous
postganglionic
neurones
to
hindlimb
during
hypothalamically
induced
vasoconstriction
and
atropine-sensitive
vasodilation.
Pfluigers
Archiv
361,
231-240.
Kocsis,
B.,
GEBBER,
G.
L.,
BARMAN,
S.
M.
&
KENNEY,
M.
J.
(1990).
Relationships
between
activity
of
sympathetic
nerve
pairs:
phase
and
coherence.
American
Journal
of
Physiology
259.
R549-560.
LUNDBERG,
J..
NORGREN,
L.,
RIBBE,
E.,
ROSEN,
I.,
STEEN,
S.,
Tti6RNE.
J.
&
WALLIN,
B.
G.
(1989).
Direct
evidence
of
active
sympathetic
vasodilatation
in
the
skin
of
the
human
foot.
Journal
of
Physiology
417,
437-446.
MCKEON,
B.
&
BURKE,
D.
(1981).
Component
of
muscle
spindle
discharge
related
to
arterial
pulse.
Journal
of
Neurophysiology
46,
788-796.
MARK,
A.
L.,
VICTOR,
R.
G.
&
NERHED,
C.
&
WALLIN,
B.
G.
(1985).
Micro-neurographic
studies
of
the
mechanisms
of
sympathetic
nerve
responses
to
static
exercise
in
humans.
Circulation
Research
57,
461-469.
PETERSON,
D.
F.,
ARMSTRONG,
R.
B.
&
LAUGHLIN,
M.
H.
(1988).
Sympathetic
neural
influences
on
muscle
blood
flow
in
rats
during
submaximal
exercise.
Journal
of
Applied
Physiology
65,
434-440.
RAY,
C.
A.,
REA,
R.
F.,
CLARY,
M.
P.
&
MARK,
A.
L.
(1990).
Static
and
dynamic
leg
exercise
decreases
muscle
sympathetic
nerve
activity.
Circulation
82,
suppl.
III,
692.
RAY,
C. A.,
REA,
R.
F.,
CLARY,
M.
P.
&
MARK,
A.
L.
(1991).
Contrasting
responses
of
arm
and
leg
muscle
sympathetic
nerve
activity
to
static leg
exercise
and
postexercise
circulatory
arrest.
FASEB
Journal
5,
A382.
SAITO,
M.,
MANO,
T.,
ABE,
H.
&
IWASE,
S.
(1986).
Responses
in
muscle
sympathetic
nerve
activity
to
sustained
hand-grips
of
different
tensions
in
humans.
European
Journal
of
Applied
Physiology
55,
493-498.
SAVARD,
G.,
STRANGE,
S.,
KIENS,
B.,
RICHTER,
E.
A.,
CHRISTENSEN,
J.
N.
&
SALTIN,
B.
(1987).
Noradrenaline
spillover
during
exercise
in
active
versus
resting
skeletal
muscle
in
man.
Acta
Physiologica
Scandinavica
131,
507-515.
SEALS,
D.
R.
(1989).
Sympathetic
neural
discharge
and
vascular
resistance
during
exercise
in
humans.
Journal
of
Applied
Physiology
66,
2472-2478.
SEALS,
D.
R.
&
ENOKA,
R.
M.
(1989).
Sympathetic
activation
is
associated
with
increases
in
EMG
during
fatiguing
exercise.
Journal
of
Applied
Physiology
66,
88-95.
SUNDL6F,
G.
&
WALLIN,
B.
G.
(1977).
Human
muscle
nerve
sympathetic
activity
at
rest.
Relationship
to
blood
pressure
and
age.
Journal
of
Physiology
272,
383-397.
VALLBO,
A.
B.,
HAGBARTH,
K.-E.,
TOREBJ6RK,
H.
E.
&
WALLIN,
B.
G.
(1979).
Somatosensory,
proprioceptive,
and
sympathetic
activity
in
human
peripheral
nerves.
Physiological
Reviews
59,
919-957.
VICTOR,
R.
G.,
BERTOCCI,
L.
A.,
PRYOR,
S.
L.
&
NUNNALLY,
R.
L.
(1988).
Sympathetic
nerve
discharge
is
coupled
to
muscle
cell
pH
during
exercise
in
humans.
Journal
of
Clinical
Investigation
82,
1301-1305.
VISSING,
S.
F.,
SCHERRER,
U.
&
VICTOR,
G.
(1989).
Relation
between
sympathetic
outflow
and
vascular
resistance
in
the
calf
during
perturbations
in
central
venous
pressure.
Circulation
Research
65,
1710-1717.
WALLIN,
B.
G.
&
FAGIUS,
J.
(1988).
Peripheral
sympathetic
neural
activity
in
conscious
humans.
Annual
Review
of
Physiology
50,
565-576.
WALLIN,
B.
G.,
VICTOR,
R.
G.
&
MARK,
A.
L.
(1989).
Sympathetic
outflow
to
resting
muscles
during
static
handgrip
and
post-contraction
muscle
ischemia.
American
Journal
of
Physiology
256,
H10S-110.
233
