
AP Chemistry Name:
Ch.1 – Matter, Measurement, and Problem Solving Date:
1.1 Atoms and Molecules
1.2 The Scientific Approach to Knowledge
1. Classify each statement as an observation, a low, or a theory. Justify your answers.
a. Chlorine is a highly reactive gas.
b. If elements are listed in order of increasing mass of their atoms, their chemical reactivity
follows a repeating pattern.
c. Neon is an inert (or nonreactive) gas.
d. The reactivity of elements depends on the arrangement of their electrons.
1.3 The Classification of Matter
1. Classify each substance as a pure substance or a mixture. If it is a pure substance, classify it as an
element or a compound. If it is a mixture, classify it as homogeneous or heterogeneous.
a. Wine
b. Beef stew
c. Iron
d. Carbon monoxide
1.4 Physical and Chemical Changes and Physical and Chemical Properties
1. Identify the following changes as physical or chemical changes
a. Baking soda reacts with vinegar to produce carbon dioxide. _____________________________
b. The copper sheath on the Statue of Liberty turns green. _____________________________
c. Addition of salt melts ice on the highway. _____________________________
d. Steam condenses on the windowpane. _____________________________
e. Epoxy resin cures and hardens. _____________________________
f. Sugar dissolves in a cup of coffee. _____________________________
g. Natural gas burns in a furnace. _____________________________
2. Which of the following physical properties are extensive?
a. heat capacity
b. viscosity
c. melting point
d. conductivity
e. specific heat capacity
f. density
1.5 Energy: A Fundamental Part of Physical and Chemical Change
1.6 The Units of Measurement
1. Convert 25°C to K.
2. Convert 350. K to °C.
3. Calculate the density of lead if a 10. kg block has a volume of 885 cm
3
.
4. What is the volume of a 100. g bar of aluminum if its density is 2.70 g·cm
-3
?
5. Calculate the mass of 100. cm
3
of uranium (density 19.07 g·cm
-3
).
6. Acetone (nail polish remover) has a density of 0.7857 g/cm
3
.
a. What is the mass, in g, of 28.56 mL of acetone?
b. What is the volume, in mL, of 6.54 g of acetone?
7. A 12.3 g block of an unknown metal is immersed in water in a graduated cylinder. The level of
water in the cylinder rose. The level of water in the cylinder rose exactly the same distance
when 17.4 grams of aluminum (density 2.70 g·cm
-3
) was added to the same cylinder. What is the
unknown metal’s density?
8. Use prefix multipliers to express each measurement without any exponents.
a. 38.8 x 10
5
g
b. 55.2 x 10
-10
s
c. 23.4 x 10
11
m
d. 87.9 x 10
-7
L
9. Use scientific notation to express each quantity with only base units (no multipliers).
a. 35 μL
b. 225 Mm
c. 133 Tg
d. 1.5 cg
1.7 The Reliability of a Measurement
1. Write the following numbers in scientific notation with the correct number of significant figure
a. 1,327
b. 0.00562
c. 2.76
d. 0.166
e. 0.09911
2. Measurements of the boiling point of a liquid were taken by two laboratory technicians (A and
B). The actual boiling point was 92.3. Which technician achieved the most accurate result and
which technician was the most precise? Explain your answer.
A: 92.0 92.1 92.4 92.2
B: 91.9 92.5 92.6 92.0
3. Evaluate the following expressions. Express the answers in scientific notation with the correct
number of significant figures and the correct units.
a. 0.0045 in + 1.0098 in + 0.987 in + 23.08 in
b. (3.45 cm
3
x 2.70 g·cm
-3
) + (7.433 cm
3
x 1.677 g·cm
-3
)
c. 2.703 g/(1.376 cm x 2.45 cm x 3.78 cm)
1.8 Solving Chemical Problems
1. Convert each of the following. Show all work.
a. 1342 mL into L
b. 3.26 x 10
-6
km into mm
c. 8,768 mg into g
d. 400 cm
3
into m
3
e. 3600 sq. in. into sq. ft.
f. 521 m into km
2. If one pound is 453.59 grams, how many grams are there in one ounce? How many ounces are
there in one kilogram? (There are 16 ounces in a pound)
3. A sample of gold alloy contains 5.6% silver by mass. How many grams of silver are there in 1
kilogram of the alloy?
Review questions: on a separate sheet of paper, write or type your answers to the following
review questions. Your answers must be in complete sentences.
Chapter 1 review questions: 8, 9, 11, 18, 19, 25, 32

AP Chemistry Name:
Ch. 2 – Atoms and Elements Date:
2.1 Imaging and Moving Individual Atoms
2.2 Early Ideas about the Building Blocks of Matter
2.3 Modern Atomic Theory and the Laws that Led to It
1. Two samples of sodium chloride were decomposed into their constituent elements. One sample
produced 6.98 g of sodium and 10.7 g of chlorine, and the other sample produced 11.2 g of
sodium and 17.3 g of chlorine. Are these results consistent with the law of definite proportions?
Explain your answer.
2. Sulfur and fluorine form several different compounds including sulfur hexafluoride and sulfur
tetrafluoride. Decomposition of a sample of sulfur hexafluoride produces 4.45 g of fluorine and
1.25 g of sulfur, while decomposition of a sample of sulfur tetrafluoride produces 4.43 g of
fluorine and 1.87 g of sulfur. Calculate the mass of fluorine per gram of sulfur for each sample
and show that these results are consistent with the laws of multiple proportions.
2.4 The Discovery of the Electron
1. To illustrate Robert Millikan’s determination of the charge on an electron, suppose that you
were given the task of determining the mass of a single jelly bean given the following
experimental data. Various scoops of jelly beans were weighed and the following masses
determined. The number of jelly beans in each scoop was not known.
Masses (in grams) of ten different scoops:
4.96
8.68
13.64
7.44
21.08
16.12
9.92
19.84
6.20
12.40
2.5 The Structure of the Atom
2.6 Subatomic Particles: Protons, Neutrons, and Electrons in Atoms
1. How many protons are found in
12
C?
13
C?
13
C
-1
?
2. How many neutrons are found in
12
C?
13
C?
13
C
-1
?
3. How many electrons are found in
12
C?
13
C?
13
C
-1
?

4. What do all carbon atoms (and ions) have in common?
5. How is the charge on an ion determined?
6. Where is most of the mass of an atom, within the nucleus or outside of the nucleus? Explain your
reasoning.
7. Complete the following table:
Isotope
Atomic
Number
Z
Mass
Number
A
Number of
electrons
31
P
15
18
O
8
19
39
18
58
Ni
2+
58
8. Give the mass number of each of the following atoms:
a. an iron atom with 30 neutrons ___________
b. an americium atom with 148 neutrons ___________
c. a tungsten atom with 110 neutrons ___________
9. Give the complete symbol (
) for each of the following atoms:
a. nitrogen with 8 neutrons _______
b. zinc with 34 neutrons _______
c. xenon with 75 neutrons _______
10. How many electrons, protons, and neutrons are there in an atom of:
a. carbon-13 electrons _______ protons _______ neutrons _______
b. copper-63 electrons _______ protons _______ neutrons _______
c. bismuth-205 electrons _______ protons _______ neutrons _______

11. Fill in the blanks in the table (one column per element).
Symbol
Number of protons
78
Number of
neutrons
117
46
Number of
electrons
in the neutral atom
36
Name of element
12. Radioactive americium-241 is used in household smoke detectors and in bone mineral analysis.
Give the number of electrons, protons, and neutrons in an atom of americium-241.
13. Copper has two stable isotopes, and, with masses of 62.939598 amu and 64.927793 amu,
respectively. Calculate the percent abundances of these isotopes of copper.
14. Which of the following atoms are isotopes of the same element? Identify the elements of these
isotopes and describe the number of protons and neutrons in the nucleus of them all.
15
7
X
12
6
X
13
7
X
18
8
X
14
7
X
14
6
X
16
8
X
13
6
X
17
8
X
15. Which of the following are isotopes of element X, with atomic number of 9?
2.7 Finding Patterns: The Periodic Law and the Periodic Table
1. Match the following
a. Sodium
b. Chlorine
c. Nickel
d. Argon
e. Calcium
f. Uranium
g. Oxygen
_______ Alkali metal
_______ Alkaline earth metal
_______ Transition metal
_______ Actinide
_______ Halogen
_______ Noble gas
_______ Chalcogen (group 6A)
2. Write the names of the following elements:
a. N _________________________________
b. Ca _________________________________
c. K _________________________________
d. P _________________________________
e. Cr _________________________________
f. V _________________________________
3. Write the symbols for the following elements
a. Silicon _________
b. Chlorine _________
c. Iron _________
d. Sodium _________
e. Silver _________
f. Sulfur ________
2.8 Atomic Mass: The Average Mass of an Element’s Atoms
1. Verify that the atomic mass of magnesium is 24.31 amu, given the following information:
Magnesium-24, mass = 23.985042 amu; percent abundance = 78.99%
Magnesium-25, mass = 24.985837 amu; percent abundance = 10.00%
Magnesium-26, mass = 25.982593 amu; percent abundance = 11.01%
2. There are three naturally occurring isotopes of neon:
neon-20 mass 19.9924 amu abundance 90.84%
neon-21 mass 20.9940 amu abundance 0.260%
neon-22 mass 21.9914 amu abundance 8.90%
a. Without calculation, what is the approximate atomic mass of neon? _____________
b. Calculate the actual atomic mass.
3. Uranium has an atomic mass equal to 238.0289. It consists of two isotopes: uranium-235 with
an isotopic mass of 235.044 amu and uranium-238 with an isotopic mass of 238.051. Calculate
the % abundance of the uranium-235 isotope.

2.9 Molar Mass: Counting Atoms by Weighing Them
1. Calculate the molar mass of each substance. Give answers to two decimal places
H
2
SO
4
Cl
2
Ca(OH)
2
HC
2
H
3
O
2
CO
2
N
2
O
NaOCl
Al
2
S
3
2. How many moles are there in 8.3 x 10
8
atoms of Zn?
3. How many atoms of Ag are contained in 73,000 grams?
4. What would be the mass of 47,000,000 atoms of O?
5. What would be the mass of 1 atom of Fe?
6. How many moles are there in 352 grams of N?
7. What is the mass of 3.98 x 10
24
H molecules?
Review questions: on a separate sheet of paper, write or type your answers to the following
review questions. Your answers must be in complete sentences.
Chapter 2 review questions: 5, 12, 20, 21, 22, 23

AP Chemistry Name:
Ch. 3 – Molecules, Compounds, and Chemical Equations Date:
3.1 Hydrogen, Oxygen, and Water
3.2 Chemical Bonds
3.3 Representing Compounds: Chemical Formulas and Molecular Models
1. The structural formula for acetic acid is CH
3
CO
2
H.
a. What is its empirical formula? __________________
b. What is its molecular formula? __________________
2. Determine the number of each type of atom in each formula.
a. Ca(NO
2
)
2
b. CuSO
4
c. Al(NO
3
)
3
d. Mg(HCO
3
)
2
3. Identify the elements that have molecules as their basic units.
a. Hydrogen b. Lead c. Iodine d. Oxygen
4. Classify each compound as ionic or molecular
a. CF
2
Cl
2
________________________________
b. CCl
4
________________________________
c. PtO
2
________________________________
d. SO
3
________________________________
3.4 An Atomic-Level View of Elements and Compounds
1. Based on the molecular views, classify each substance as an atomic element, a molecular
element, an ionic compound, or a molecular compound.

3.5 Ionic Compounds: Formulas and Names
3.6 Molecular Compounds: Formulas and Names
3.7 Summary of Inorganic Nomenclature
1. Name the polyatomic ions.
a. CH
3
CO
2
-
__________________
b. H
2
PO
4
-
__________________
c. SO
3
2-
__________________
d. HCO
3
-
__________________
e. Cr
2
O
7
2-
__________________
f. ClO
4
-
_________________
2. What are the formulas of the polyatomic ions?
a. Phosphate ___________
b. Nitrite ___________
c. Sulfate ___________
d. Cyanide ___________
e. Bisulfite ___________
f. Chlorite ___________
3. Writing Ionic Formulas
Cl
-
NO
3
-
S
2-
CO
3
2-
N
3-
PO
4
3-
OH
-
Na
+
NH
4
+
Sn
2+
Hg
2
2+
Al
3+
Sn
4+
4. Naming Ionic Compounds
Cation
Anion
Formula
Name
Cu
2+
OH
-
Ba
2+
SO
4
2-
NH
4
+
Cr
2
O
7
2-
Ag
+
C
2
H
3
O
2
-
Fe
3+
S
2-
5. Write the number that corresponds with each prefix.
mono
di
tri
tetra
penta
hexa
hepta
octa
nona
deca
Note that sections
3.5-3.7 are grouped
together
6. Writing Formulas of Binary Nonmetal Compounds
Name
Formula
Name
Formula
nitrogen trifluoride
phosphorus trichloride
nitrogen monoxide
phosphorus
pentachloride
nitrogen dioxide
sulfur hexafluoride
dinitrogen tetroxide
disulfur decafluoride
dinitrogen monoxide
xenon tetrafluoride
7. Naming Binary Nonmetal Compounds
Name
Formula
Name
Formula
CCl
4
HBr
P
4
O
10
N
2
F
4
ClF
3
XeF
3
BCl
3
PI
3
SF
4
SCl
2
8. Practice for Both Types of Compounds
Formula
Name
Formula
Name
HCl
carbon dioxide
PCl
5
ammonium carbonate
K
2
S
sulfur dichloride
NiSO
4
calcium iodide
ClF
3
boron trifluoride
OF
2
phosphorus triiodide
Al(OH)
3
magnesium perchlorate
NCl
3
potassium permanganate
(NH
4
)
3
PO
4
aluminum phosphate
S
2
Cl
2
dioxygen difluoride
9. Write the ions present in the following salts and predict their formulas:
Cation (+)
Anion (-)
Formula
potassium bromide
K
+
Br
-
KBr
calcium carbonate
magnesium iodide

Cation (+)
Anion (-)
Formula
lithium oxide
aluminum sulfate
ammonium chlorate
beryllium phosphate
10. Name the following ionic salts
a. (NH
4
)
2
SO
4
________________________________
b. KHCO
3
________________________________
c. Ca(NO
3
)
2
________________________________
d. Co
2
(SO
4
)
3
________________________________
e. NiSO
4
________________________________
f. AlPO
4
________________________________
11. Name the following binary compounds of the nonmetals
a. CS
2
__________________________________
b. SF
6
__________________________________
c. IF
5
__________________________________
d. N
2
H
4
__________________________________
e. PCl
5
__________________________________
f. Cl
2
O
7
__________________________________
g. SiCl
4
__________________________________
h. GeH
4
__________________________________
i. P
4
O
10
__________________________________
j. S
4
N
4
__________________________________
k. OF
2
__________________________________
l. IF
7
__________________________________
12. What are the formulas for the following binary compounds?
a. silicon dioxide ________________
b. boron trifluoride ________________
c. xenon tetroxide ________________
d. dinitrogen pentoxide ________________
e. bromine trifluroide ________________
f. carbon tetrachloride ________________
g. phosphine ________________
h. silicon carbide ________________
i. disulfur dichloride ________________
j. hydrogen selenide ________________
3.8 Formula Mass and the Mole Concept for Compounds
Show all work and include units in your final answer.
1. How many moles are present in 128 grams of sulfur dioxide?
2. What is the mass of 3 moles of oxygen molecules?
3. If 5 moles of a metallic element have a mass of 200 grams, which element is it?
4. What is the molar mass of methane CH
4
?
5. What is the mass of 9 moles of fluorine molecules?
6. 102 grams of a gas contains 6 moles. What is its molar mass?
7. How many grams are there in one mole of benzene C
6
H
6
?
8. How many moles of nitrogen atoms are there in 6 moles of TNT (CH
3
C
6
H
2
(NO
2
)
3
)?
9. What is the molar mass of TNT?

3.9 Composition of Compounds
Determine the percent composition of each element below:
1. H
2
SO
4
H
S
O
2. Ca(OH)
2
Ca
O
H
3. HC
2
H
3
O
2
H
C
O
4. CO
2
C
O
5. N
2
O
N
O
6. NaOCl
Na
O
Cl
7. Al
2
S
3
Al
S
3.10 Determining a Chemical Formula from Experimental Data
1. Cupric chloride, CuCl
2
, when heated to 100°C is dehydrated. If 0.235 g of CuCl
2
· x H
2
O gives
0.185 g of CuCl
2
on heating, what is the value of x?
2. The “alum” used in cooking is potassium aluminum sulfate hydrate, KAl(SO
4
)
2
· x H
2
O . To find
the value of x, you can heat a sample of the compound to drive off all of the water and leave only
KAl(SO
4
)
2
. Assume you heat 4.74 g of the hydrated compound and that the sample loses 2.16 g
of water. What is the value of x?
3. If “Epsom salt,” MgSO
4
· x H
2
O is heated to 250 C, all the water of hydration is lost. On heating a
1.687-g sample of the hydrate, 0.824 g of MgSO
4
remains. What is the formula of Epsom salt?
4. When CaSO
4
· x H
2
O is heated, all of the water is driven off. If 34.0 g of CaSO
4
(molar mass = 136)
is formed from 43.0 g of CaSO
4
· x H
2
O, what is the value of x?
5. The hydrocarbons ethylene (molar mass 28 g/mol), cyclobutane (molar mass 56 g/mol),
pentene (molar mass 70 g/mol), and cyclohexane (molar mass 84 g/mol), all have the same
empirical formula. What is it? Write the molecular formulas for these four compounds.
6. A compound was analyzed and found to contain 76.57% carbon, 6.43% hydrogen, and 17.00%
oxygen by mass. Calculate the empirical formula of the compound. If the molar mass of the
compound is 94.11g/mol, what is the molecular formula of the compound?
7. A compound was analyzed and found to contain 53.30% carbon, 11.19% hydrogen, and 35.51%
oxygen by mass. Calculate the empirical formula of the compound. If the molar mass of the
compound is 90.12 g/mol, what is the molecular formula of the compound?
8. Combustion analysis of naphthalene, a hydrocarbon used in mothballs, produced 8.80 g carbon
dioxide and 1.44 g water. Calculate the empirical formula for naphthalene
9. Tartaric acid is the white, pwdery substance that coats tart candies such as Sour Patch Kids.
Combustion analysis of a 12.02 g sample of tartaric acid (which contains only C, H, and O)
produced 14.08 g of carbon dioxide and 4.32 grams of water. Determine the empirical formula of
tartaric acid.
3.11 Writing and Balancing Chemical Equations
1. Balance the following equations:
a. __C
4
H
6
(g) + __O
2
(g) → __CO
2
(g) + __H
2
O(l)
b. __NH
3
(g) + __O
2
(g) → __NO
2
(g) + __H
2
O(l)
c. __PCl
3
(l) + __H
2
O(l) → __H
3
PO
3
(aq) + __HCl(aq)
d. __Ca
3
P
2
(s) + __H
2
O(l) → __Ca(OH)
2
(aq) + __PH
3
(g)
e. __C
4
H
8
(OH)
2
(l) + __O
2
(g) → __CO
2
(g) + __H
2
O(l)
f. __NH
3
(g) + __NO(g) → __N
2
(g) + __H
2
O(l)
g. __KClO
3
(s) → __KCl(s) + __O
2
(g)
h. __Ca(OH)
2
(s) + __H
3
PO
4
(aq) → __Ca
3
(PO
4
)
2
(s) + __H
2
O(l)
i. __C
3
H
8
(g) + __O
2
(g) → __CO
2
(g) + __H
2
O(l)
j. __N
2
O(g) + __O
2
(g) → __NO
2
(g)
k. __Al
4
C
3
(s) + _H
2
O(l) → __Al(OH)
3
(aq) + __CH
4
(g)
l. __CS
2
(l) + __Cl
2
(g) → __CCl
4
(l) + __S
2
Cl
2
(l)
m. __C
2
H
5
OH(l) + __PCl
3
(l) → __C
2
H
5
Cl(l) + __H
3
PO
3
(l)
n. __ZnS(s) + __O
2
(g) → __ZnO(s) + __SO
2
(g)
2. When asked to balance the equation C
2
H
6
(g) + O
2
(g) → CO
2
(g) + H
2
O(l) the following
suggestions were made:
a. C
2
H
6
(g) + 5O
2
(g) → 2CO
2
(g) + 3H
2
O(l)
b. C
2
H
6
(g) + 5O(g) → 2CO(g) + 3H
2
O(l)
c. 2C
2
H
6
(g) + 7O
2
(g) → 4CO
2
(g) + 6H
2
O(l)
Which answer is correct and what is wrong with each of the others?
3. Write balanced chemical equations for the following reactions
a. The decomposition of ammonium nitrate to nitrogen gas, oxygen gas, and water vapor.
b. The reaction of sodium bicarbonate with sulfuric acid to produce sodium sulfate, water,
and carbon dioxide.
c. The treatment of phosphorus pentachloride with water to produce phosphoric acid and
hydrogen chloride.
3.12 Organic Compounds
Review questions: on a separate sheet of paper, write or type your answers to the following
review questions. Your answers must be in complete sentences.
Chapter 3 review questions: 2, 5, 8, 15, 17

AP Chemistry Name:
Ch. 4 – Chemical Quantities and Aqueous Reactions Date:
4.1 Climate Change and the Combustion of Fossil Fuels
4.2 Reaction Stoichiometry: How Much Carbon Dioxide?
1. If the maximum amount of product possible is formed in the following reactions, what mass of
the specified product would you obtain?
a. 10 grams of sodium chloride is treated with excess silver nitrate
AgNO
3
(aq) + NaCl(aq) → AgCl(s) + NaNO
3
(aq)
How much silver chloride is precipitated?
b. 12 grams copper metal is treated with excess dilute nitric acid:
3Cu(s) + 8HNO
3
(aq) → 3Cu(NO
3
)
2
(aq) + 2NO(g) + 4H
2
O(l)
How much nitric oxide gas (NO) is produced?
c. 60 grams propane gas is burned in excess oxygen:
C
3
H
8
(g) + 5O
2
(g) → 3CO
2
(g) + 4H
2
O(l)
How much water is produced?
4.3 Limiting Reactant, Theoretical Yield, and Percent Yield
1. Hydrazine reacts with dinitrogen tetroxide according to the equation:
2N
2
H
4
(g) + N
2
O
4
(g) → 3N
2
(g) + 4H
2
O(g) 50.0 grams of hydrazine is mixed with 100.0
grams of dinitrogen tetroxide. How much nitrogen gas was produced?
2. Sodium metal reacts vigorously with water to produce a solution of sodium hydroxide and
hydrogen gas:
2Na(s) + 2H
2
O(l) → 2NaOH(aq) + H
2
(g) What mass of hydrogen gas can be produced
when 10 grams of sodium is added to 15 grams of water?
3. Nitrous oxide reacts with oxygen to produce nitrogen dioxide according to the equation:
2N
2
O(g) + 3O
2
(g) → 4NO
2
(g) What mass of nitrogen dioxide can be made from 42 grams of
nitrous oxide and 42 grams of oxygen?
4. If only 75 grams of nitrogen dioxide was produced in the reaction described in the previous
question, what was the percent yield?
5. Freddie flask has 4.5 g of sodium hydroxide and 3.45 x 10
23
molecules of hydrogen chloride and
wants to predict how much sodium chloride he can make according to:
___HCl + ___NaOH→___ NaCl + ____H
2
O
6. How many moles of H
2
O form when 16.9 grams of N
2
gas form according to the following
equation? 3 CuO + 2 NH
3
→ 3 Cu + 3 H
2
O + 1 N
2
7. What mass of HF must react to form 23.5 grams of H
2
O according to the following equation?
___SiO
2
+ ___HF ---> ___H
2
O + ___SiF
4
?
8. How many moles of Pb are formed when 38.2 grams of PbO react according to the following
equation? ___PbS + ___PbO ---> ___Pb + ____SO
2
?
9. How many moles of NaCl form when 7.4 moles of NaClO react according to the following
equation?
3 NaClO → 2 NaCl + 1 NaClO
3
?
10. What is the limiting reactant for the following reaction if I have 10. grams of Pb(SO
4
)
2
and 5.0
grams of LiNO
3
___Pb(SO
4
)
2
+ ___LiNO
3
___Pb(NO
3
)
4
+ ___ Li
2
SO
4
Review questions: on a separate sheet of paper, write or type your answers to the following
review questions. Your answers must be in complete sentences.
Chapter 4 review questions: 1, 2, 3

AP Chemistry
Reference: Significant Figures
The significant figures are the digits in a number which represent the accuracy of that number. All non-zero
digits in a number are significant. But zeros may be just "place holders". The following two examples show the use
of place holders in numbers.
.085 This number has an accuracy of two significant figures. In this number the "8" and "5" are measured
digits and are therefore significant. The zero is just a place holder that shows the position of the decimal point;
it is not a significant figure.
400 This number has an accuracy of one significant figure. Trailing zeros are often only place holders. In
this number the zeros are there to show that the "4" is in the hundreds column. Since no decimal point is
shown, the zeros have not been measured and are not significant.
Rules for Determining Significant Figures
1. All non-zero digits are significant.
2. Zeros to the left of non-zero digits are NEVER significant.
3. Zeros between non-zero digits are ALWAYS significant.
4. Zeros to the right of non-zero digits are significant ONLY if a decimal point is
shown.
*Notice that the terms left, between and right refer to the placement of the zeros in relationship with non-zero
numbers NOT in relationship with the decimal point.
The following examples illustrate the rules shown above as they apply to zeros:
rule 2 rule 3 rule 4
number sig figs number sig figs number sig figs
007 1 408 3 600 1
.025 2 7.002 4 8,500 2
0.09 1 30.7 3 30.0 3
.0081 2 50,009 5 46,000. 5

AP Chemistry
Reference: Significant Figure Math
When Adding or Subtracting
The answer must be rounded off to the same
column (ones, tenths, hundredths, etc.) as the
least precise measurement used in the calculation.
When Multiplying or Dividing
The answer must be rounded off to the same
number of significant figures as the least
accurate measurement used in the calculation.
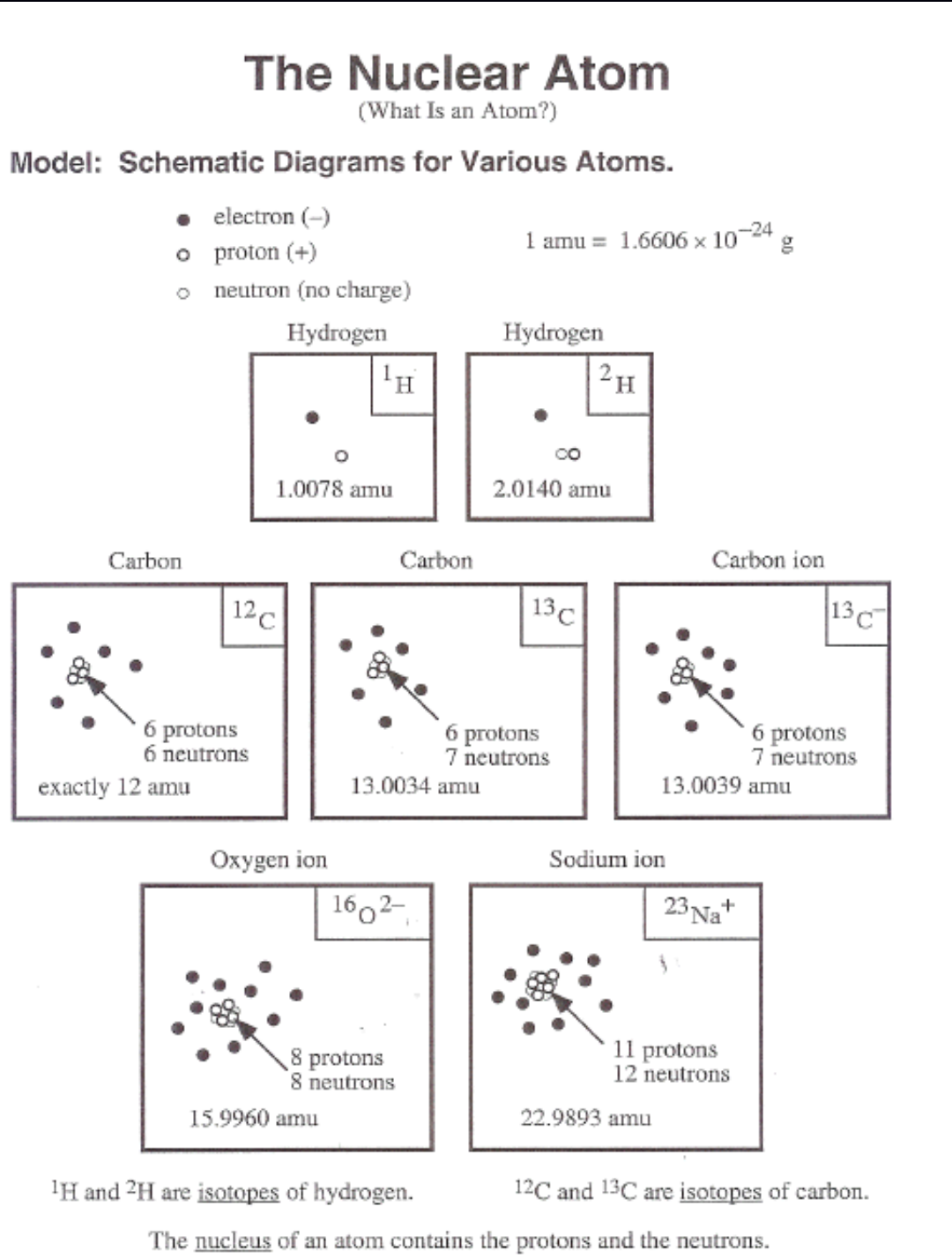
AP Chemistry
Reference: The Nuclear Atom Schematic
AP Chemistry
Reference: Writing Formulas and Naming Compounds
Introduction
Writing formulas and naming compounds can be confusing because there are different types of compounds that
follow different rules. Additionally, some compounds (H
2
O, NH
3
, CH
4
, etc.) simply have common names that must
be memorized.
The two types of compounds we will focus on first are ionic compounds (formed from positive and negative ions)
and binary nonmetal compounds (molecular compounds). Later we will add acids. So… you must recognize the
type of compound before you try to name it. [Note: + ion = “cation” and – ion = “anion”.]
Ionic
Binary Nonmetal
Formula
positive ion before negative ion
ex: NaCl (NH
4
)
2
SO
4
Al
2
S
3
usually the less electronegative atom is first
ex: CO CO
2
N
2
O
Naming
Name of cation + name of anion
sodium chloride
ammonium sulfate
aluminum sulfide
Indicate the number (mono, di, tri) and kind of atoms.
First element is simply name of element. Second
element name ends with “ide”
carbon monoxide
carbon dioxide
dinitrogen monoxide

AP Chemistry
Reference: Ions to Memorize
**note that there is some overlap on this chart with the other one, but they are not identical. You are responsible for ALL
ions listed**
aluminum
Al
3+
strontium
Sr
2+
ammonium
NH
4
+
stannous
Sn
2+
barium
Ba
2+
stannic
Sn
4+
calcium
Ca
2+
zinc
Zn
2+
cuprous
Cu
+
acetate
C
2
H
3
O
2
-
or CH
3
COO
-
cupric
Cu
2+
bromide
Br
-
ferrous
Fe
2+
carbonate
CO
3
2-
ferric
Fe
3+
chlorate
ClO
3
-
hydrogen
H
+
chloride
Cl
-
hydronium
H
3
O
+
chromate
CrO
4
2-
lead
Pb
2+
dichromate
Cr
2
O
7
2-
lithium
Li
+
fluoride
F
-
magnesium
Mg
2+
hydroxide
OH
-
manganese
Mn
2+
iodide
I
-
mercurous
Hg
2
2+
nitrate
NO
3
-
mercuric
Hg
2+
oxide
O
2-
nickel
Ni
2+
permanganate
MnO
4
-
potassium
K
+
phosphate
PO
4
3-
silver
Ag
+
sulfate
SO
4
2-
sodium
Na
+
sulfide
S
2-

AP Chemistry
Reference: Ions to Memorize
**note that there is some overlap on this chart with the other one, but they are not identical. You are responsible for ALL
ions listed**
P
3-
phosphide
PO
3
3-
phosphite
PO
4
3-
phosphate
HPO
4
2-
monohydrogen
phosphate
H
2
PO
4
-
dihydrogen
phosphate
O
2
2-
peroxide
CN
-
cyanide
SCN
-
thiocyanate
C
4-
carbide
SiO
3
2-
silicate
C
2
O
4
2-
oxalate
IO
3
-
iodate
C
2
H
3
O
2
-
acetate
H
-
hydride
OH
-
hydroxide
CrO
4
2-
chromate
Cr
2
O
7
2-
dichromate
N
3-
nitride
NO
3
-
nitrate
NO
2
-
nitrite
As
3-
arsenide
Br
-
bromide
F
-
fluoride
I
-
Iodide
CO
3
2-
carbonate
HCO
3
-
hydrogen
carbonate
(bicarbonate)
MnO
4
-
permanganate
NH
4
+
ammonium
S
2-
sulfide
HS
-
hydrogen sulfide
(bisulfide)
SO
4
2-
Sulfate
SO
3
2-
Sulfite
HSO
4
-
hydrogen sulfate
(bisulfate)
HSO
3
-
hydrogen sulfite
(bisulfite)
S
2
O
3
2-
thiosulfate
O
2-
oxide
Se
2-
selenide
Te
2-
telluride
Cations (other than
group 1A, 2A) that are
normally written
without roman
numeral charge
designators
Al
3+
Aluminum
C
4
+
carbon
Ga
3+
gallium
Si
4+
silicon
Ag
+
silver
Cl
-
chloride
ClO
4
-
perchlorate
ClO
3
-
chlorate
ClO
2
-
chlorite
ClO
-
hypochlorite

AP Chemistry
Reference: Mole Conversions
1. Given moles, find grams. Example: How many grams are in 2.3 moles of water?
2. Given moles, find atoms of a pure substance. Example: How many atoms are in 5.6 moles of carbon?
3. Given moles, find molecules of a compound. Example: how many molecules are in 3.4 moles of water?
4. Given moles, find atoms of a compound. Example: how many atoms are in 3.4 moles of water?
5. Given grams, find moles. Example: How many moles are in 45.6 grams of calcium chloride?
6. Given grams, find atoms (of a pure substance) or molecules (of a compound). Example: how many atoms
are in 75.6 grams of sodium? How many molecules are in 65.4 grams of sodium chloride?
7. Given atoms (of a pure substance) or molecules (of a compound) find moles. Example: how many moles are
in 5.45 x 10
23
carbon atoms? How many moles are in 3.78 x 10
23
water molecules?
8. Given atoms (of a pure substance) or molecules (of a compound), find grams. Example: what is the mass of
4.56 x 10
23
carbon atoms? What is the mass of 3.45 x 10
24
water molecules?
