
Pre-AP
®
Chemistry
COURSE GUIDE
INCLUDES
Approach to
teaching and
learning
Course map
Course framework
Sample
assessment
questions
preap.org/Chemistry-CG
© 2021 College Board. 01560-064
01560-064-Pre-AP-Covers-3P.indd 79-80 3/27/20 1:38 PM

ABOUT COLLEGE BOARD
College Board is a mission-driven not-for-prot organization that connects students to college
success and opportunity. Founded in 1900, College Board was created to expand access
to higher education. Today, the membership association is made up of over 6,000 of the
world’s leading educational institutions and is dedicated to promoting excellence and equity
in education. Each year, College Board helps more than seven million students prepare for
a successful transition to college through programs and services in college readiness and
college success—including the SAT
®
and the Advanced Placement Program
®
. The organization
also serves the education community through research and advocacy on behalf of students,
educators, and schools.
For further information, visit www.collegeboard.org.
College Board believes thatallstudents deserve engaging, relevant, and challenging grade-
level coursework. Access to this type of coursework increases opportunities for all students,
including groups that have been traditionally underrepresented in AP and college classrooms.
Therefore, the Pre-AP program is dedicated to collaborating with educators across the country
to ensure all students have the supports to succeed in appropriately challenging classroom
experiences that allow students to learn and grow. It is only through a sustained commitment to
equitable preparation, access, and support that true excellence can be achieved for all students,
and the Pre-AP course designation requires this commitment.
ISBN: 978-1-4573-1486-5
© 2021 College Board. PSAT/NMSQT is a registered trademark of the College Board and National Merit
Scholarship Corporation.
1 2 3 4 5 6 7 8 9 10

Contents
v Acknowledgments
ABOUT PRE-AP
3 Introduction to Pre-AP
3 Developing the Pre-AP Courses
3 Pre-AP Educator Network
4 How to Get Involved
5 Pre-AP Approach to Teaching and Learning
5 Focused Content
5 Horizontally and Vertically Aligned Instruction
8 Targeted Assessments for Learning
9 Pre-AP Professional Learning
ABOUT PRE-AP CHEMISTRY
13 Introduction to Pre-AP Chemistry
13 Pre-AP Science Areas of Focus
15 Pre-AP Chemistry and Career Readiness
16 Summary of Resources and Supports
18 Course Map
20 Pre-AP Chemistry Course Framework
20 Introduction
21 Course Framework Components
22 Big Ideas in Pre-AP Chemistry
23 Overview of Pre-AP Chemistry Units and Enduring Understandings
24 Unit 1: Structure and Properties of Matter
28 Unit 2: Chemical Bonding and Interactions
33 Unit 3: Chemical Quantities
36 Unit 4: Chemical Transformations
42 Pre-AP Chemistry Model Lessons
43 Support Features in Model Lessons
44 Pre-AP Chemistry Assessments for Learning
44 Learning Checkpoints
46 Performance Tasks
48 Sample Performance Task and Scoring Guidelines
63 Final Exam
64 Sample Assessment Questions
69 Pre-AP Chemistry Course Designation
71 Accessing the Digital Materials
Acknowledgments
College Board would like to acknowledge the following committee members, consultants, and
reviewers for their assistance with and commitment to the development of this course. All
individuals and their aliations were current at the time of their contribution.
Roxie Allen, St. John’s School, Houston, TX
Kristen Cacciatore, Charlestown High School, Boston, MA
Michael Diaz, Achievement First, New Haven, CT
Kristen Drury, William Floyd High School, Mastic Beach, NY
Amy Earle, Deep Run High School, Richmond, VA
Ryan Johnson, Doherty High School, Colorado Springs, CO
Dena Leggett, Franklin High School, Franklin, TN
Paul Price, Trinity Valley School, Fort Worth, TX
Kaleb Underwood, Education Consultant, Charlottesville, VA
Fred Vital, Darien High School, Darien, CT
David Yaron, Carnegie Mellon University, Pittsburgh, PA
COLLEGE BOARD STAFF
Laura Casdorph, Director, Pre-AP Chemistry Curriculum, Instruction, and Assessment
Karen Lionberger, Senior Director, Pre-AP STEM Curriculum, Instruction, and Assessment
Beth Hart, Senior Director, Pre-AP Assessment
Mitch Price, Director, Pre-AP STEM Assessment
Natasha Vasavada, Executive Director, Pre-AP Curriculum, Instruction, and Assessment

About Pre-AP


3
About Pre-AP
Introduction to Pre-AP
Every student deserves classroom opportunities to learn, grow, and succeed. College
Board developed Pre-AP® to deliver on this simple premise. Pre-AP courses are
designed to support all students across varying levels of readiness. They are not honors
or advanced courses.
Participation in Pre-AP courses allows students to slow down and focus on the most
essential and relevant concepts and skills. Students have frequent opportunities
to engage deeply with texts, sources, and data as well as compelling higher-order
questions and problems. Across Pre-AP courses, students experience shared
instructional practices and routines that help them develop and strengthen the
important critical thinking skills they will need to employ in high school, college, and
life. Students and teachers can see progress and opportunities for growth through
varied classroom assessments that provide clear and meaningful feedback at key
checkpoints throughout each course.
Pre-AP courses are carefully developed in partnership with experienced educators,
including middle school, high school, and college faculty. Pre-AP educator committees
work closely with College Board to ensure that the course resources define, illustrate,
and measure grade-level-appropriate learning in a clear, accessible, and engaging way.
College Board also gathers feedback from a variety of stakeholders, including Pre-AP
partner schools from across the nation who have participated in multiyear pilots of
select courses. Data and feedback from partner schools, educator committees, and
advisory panels are carefully considered to ensure that Pre-AP courses provide all
students with grade-level-appropriate learning experiences that place them on a path to
college and career readiness.
Similar to the way in which teachers of Advanced Placement® (AP®) courses can
become more deeply involved in the program by becoming AP Readers or workshop
consultants, Pre-AP teachers also have opportunities to become active in their
educator network. Each year, College Board expands and strengthens the Pre-AP
National Faculty—the team of educators who facilitate Pre-AP Readiness Workshops
and Pre-AP Summer Institutes. Pre-AP teachers can also become curriculum and
assessment contributors by working with College Board to design, review, or pilot the
course resources.

4
Course Guide
© 2021 College Board
Pre-AP Chemistry
Introduction to Pre-AP
About Pre-AP
Schools and districts interested in learning more about participating in Pre-AP should
visit preap.collegeboard.org/join or contact us at p[email protected].
Teachers interested in becoming members of Pre-AP National Faculty or participating
in content development should visit preap.collegeboard.org/national-faculty or
contact us at preap@collegeboard.org.

5
About Pre-AP
Pre-AP Approach to Teaching and Learning
Pre-AP courses invite all students to learn, grow, and succeed through focused content,
horizontally and vertically aligned instruction, and targeted assessments for learning.
The Pre-AP approach to teaching and learning, as described below, is not overly
complex, yet the combined strength results in powerful and lasting benefits for both
teachers and students. This is our theory of action.
Focused Content
Course Frameworks,
Model Lessons
Horizontally and
Vertically Aligned
Instruction
Shared Principles,
Areas of Focus
Targeted Assessments
and Feedback
Learning Checkpoints,
Performance Tasks,
Final Exam
Pre-AP courses focus deeply on a limited number of concepts and skills with the
broadest relevance for high school coursework and college and career success. The
course framework serves as the foundation of the course and defines these prioritized
concepts and skills. Pre-AP model lessons and assessments are based directly on this
focused framework. The course design provides students and teachers with intentional
permission to slow down and focus.
Shared principles cut across all Pre-AP courses and disciplines. Each course is also
aligned to discipline-specific areas of focus that prioritize the critical reasoning skills
and practices central to that discipline.

6
Course Guide
© 2021 College Board
Pre-AP Chemistry
Pre-AP Approach to Teaching and Learning
About Pre-AP
All Pre-AP courses share the following set of research-supported instructional
principles. Classrooms that regularly focus on these cross-disciplinary principles allow
students to effectively extend their content knowledge while strengthening their critical
thinking skills. When students are enrolled in multiple Pre-AP courses, the horizontal
alignment of the shared principles provides students and teachers across disciplines
with a shared language for their learning and investigation, and multiple opportunities
to practice and grow. The critical reasoning and problem-solving tools students
develop through these shared principles are highly valued in college coursework and in
the workplace.
Close Observation
and Analysis
Higher-Order
Questioning
Academic
Conversation
Evidence-Based
Writing
SHARED
PRINCIPLES
Close Observation and Analysis
Students are provided time to carefully observe one data set, text, image, performance
piece, or problem before being asked to explain, analyze, or evaluate. is creates
a safe entry point to simply express what they notice and what they wonder. It also
encourages students to slow down and capture relevant details with intentionality to
support more meaningful analysis, rather than rushing to completion at the expense
of understanding.
Students engage with questions designed to encourage thinking that is elevated
beyond simple memorization and recall. Higher-order questions require students to
make predictions, synthesize, evaluate, and compare. As students grapple with these
questions, they learn that being inquisitive promotes extended thinking and leads to
deeper understanding.

7
Pre-AP Approach to Teaching and Learning
About Pre-AP
With strategic support, students frequently engage in writing coherent arguments
from relevant and valid sources of evidence. Pre-AP courses embrace a purposeful
and scaolded approach to writing that begins with a focus on precise and eective
sentences before progressing to longer forms of writing.
Academic Conversation
rough peer-to-peer dialogue, students’ ideas are explored, challenged, and rened.
As students engage in academic conversation, they come to see the value in being
open to new ideas and modifying their own ideas based on new information. Students
grow as they frequently practice this type of respectful dialogue and critique and learn
to recognize that all voices, including their own, deserve to be heard.
AREAS OF FOCUS
The areas of focus are discipline-specific reasoning skills that students develop
and leverage as they engage with content. Whereas the shared principles promote
horizontal alignment across disciplines, the areas of focus provide vertical alignment
within a discipline, giving students the opportunity to strengthen and deepen their
work with these skills in subsequent courses in the same discipline.
Arts
English
Mathematics
Science
Social Studies
Areas of Focus
Align Vertically Within Disciplines
(Grades 6-12)
Shared Principles
Align Horizontally Across All Courses
Academic Conversation
Higher-Order Questioning
Evidence-Based Writing
Close Observation and Analysis
For information about the Pre-AP science areas of focus, see page 13.

8
Course Guide
© 2021 College Board
Pre-AP Chemistry
Pre-AP Approach to Teaching and Learning
About Pre-AP
Pre-AP courses include strategically designed classroom assessments that serve as
tools for understanding progress and identifying areas that need more support. The
assessments provide frequent and meaningful feedback for both teachers and students
across each unit of the course and for the course as a whole. For more information
about assessments in Pre-AP Chemistry, see page 44.

9
About Pre-AP
Pre-AP Professional Learning
The summer before their first year teaching a Pre-AP course, teachers are required
to engage in professional learning offered by College Board. There are two options
to meet this requirement: the Pre-AP Summer Institute (Pre-APSI) and the Online
Foundational Module Series. Both options provide continuing education units to
educators who complete the training.
The Pre-AP Summer Institute is a four-day collaborative experience that empowers
participants to prepare and plan for their Pre-AP course. While attending, teachers
engage with Pre-AP course frameworks, shared principles, areas of focus, and
sample model lessons. Participants are given supportive planning time where they
work with peers to begin to build their Pre-AP course plan.
The Online Foundational Module Series will be available beginning July 2020 to
all teachers of Pre-AP courses. These 12- to 20-hour courses will support teachers
in preparing for their Pre-AP course. Teachers will explore course materials and
experience model lessons from the student’s point of view. They will also begin
to plan and build their own course materials, so they are ready on day one of
instruction.
Pre-AP teachers also have access to the Online Performance Task Scoring Modules,
which offer guidance and practice applying Pre-AP scoring guidelines to student work.

About Pre-AP
Chemistry


13
About Pre-AP Chemistry
Introduction to Pre-AP Chemistry
The Pre-AP Chemistry course emphasizes the integration of content with science
practices—powerful reasoning tools that support students in analyzing the natural
world around them. Having this ability is one of the hallmarks of scientific literacy and
is critical for numerous college and career endeavors in science and the social sciences.
Rather than seeking to cover all topics traditionally included in a standard chemistry
textbook, this course focuses on the foundational chemistry knowledge and skills
that matter most for college and career readiness. The Pre-AP Chemistry Course
Framework highlights how to guide students to connect core ideas within and across
the units of the course, promoting the development of a coherent understanding of
matter at the atomic scale.
The components of this course have been crafted to prepare not only the next
generation of chemists, but also a broader base of chemistry-informed citizens who are
well equipped to respond to the array of science-related issues that impact our lives at
the personal, local, and global levels.
The Pre-AP science areas of focus, shown below, are science practices that students
develop and leverage as they engage with content. They were identified through
educator feedback and research about where students and teachers need the most
curriculum support. These areas of focus are vertically aligned to the science practices
embedded in other science courses in high school, including AP, and in college, giving
students multiple opportunities to strengthen and deepen their work with these skills
throughout their educational career. They also support and align to the NGSS and AP
science practices of theory building and refinement.
Attention
to
Modeling
Strategic Use of
Mathematics
Emphasis
on Analytical
Reading and
Writing
Science
Areas of Focus
14
Course Guide
© 2021 College Board
Pre-AP Chemistry
Introduction to Pre-AP Chemistry
About Pre-AP Chemistry
Students engage in analytical reading and writing to gain, retain, and apply
scientic knowledge and to carry out scientic argumentation.
In prioritizing analytical reading, Pre-AP Chemistry classrooms ask students to
extract, synthesize, and compare complex information, oen by moving between
texts, tables and graphs of experimental data, and representations of motions and
interactions at the molecular level. rough analytical writing activities, Pre-AP
Chemistry students must integrate and translate that information to generate scientic
questions, design methods for answering questions, and develop scientic arguments.
Moreover, the application of these skills to the understanding of informal science
texts, such as articles found in newspapers, online sources, and magazines, prepares
students to be discerning consumers of scientic information.
Strategic Use of Mathematics
Students integrate mathematics with conceptual understanding to model chemical
phenomena.
Mathematics is an essential tool for the study of chemistry. However, introductory
chemistry courses oen focus on the use of mathematics without context-focused
applications. is practice can result in students being able to solve mathematical
problems in chemistry class, but without an understanding of the underlying chemical
principles. As an alternative approach, Pre-AP Chemistry requires students to
demonstrate their knowledge using multiple representations that integrate conceptual
understanding with the use of mathematics. Students are also challenged to use
data and observations to build mathematical models that reect their conceptual
understanding and can be used to make predictions.
Attention to Modeling
Students develop and rene models to connect macroscopic observations to
structure, motion, and interactions occurring at the atomic scale.
In Pre-AP Chemistry, the development of models to explain their macroscopic
observations is a primary means through which students develop an understanding
of the molecular world. Engaging students in creating and revising models reinforces
other scientic reasoning skills, such as data analysis and scientic argumentation.
Modeling also helps illustrate for students how scientic knowledge is constructed and
modied over time as new data and evidence emerge and models are revised based on
this new information.

15
Introduction to Pre-AP Chemistry
About Pre-AP Chemistry
The Pre-AP Chemistry course resources are designed to expose students to a wide range of
career opportunities that depend upon chemistry knowledge and skills. Chemistry lies at
the interface of the physical and life sciences. Asscience,engineering, and healthcaremove
increasingly towards the molecular scale, chemistry provides ideal preparation for 21st
century careers.Examples include not only careers within the physical sciences, such as
forensic scientist or food chemist, but also other endeavors where chemistry knowledge is
relevant such as the work of an engineer, policymaker, or healthcare worker.
Career clusters that involve chemistry, along with examples of careers in chemistry or
related to chemistry, are provided below. Teachers should consider discussing these
with students throughout the year to promote motivation and engagement.
Career Clusters Involving Chemistry
agriculture, food, and natural resources
healthcare and health science
hospitality and tourism
information technology
manufacturing
STEM (science, technology, engineering,
and math)
Examples of Chemistry Careers Examples of Chemistry Related Careers
atmospheric chemist
chemical engineer
chemistry teacher/professor
environmental chemist
food chemist
geochemist
hazardous waste manager
materials scientist
medicinal chemist
nanotechnologist
synthetic chemist
environmental scientist
forensic scientist
medical assistant
patent lawyer
pharmacist
pharmacologist
physician
physician assistant
science writer
technical sales
toxicologist
Source for Career Clusters: “Advanced Placement and Career and Technical Education: Working Together.”
Advance CTE and the College Board. October 2018. https://careertech.org/resource/ap-cte-working-
together.
For more information about careers that involve chemistry, teachers and students can
visit and explore the College Board’s Big Future resources:
https://bigfuture.collegeboard.org/majors/physical-sciences-chemistry-chemistry.

16
Course Guide
© 2021 College Board
Pre-AP Chemistry
Introduction to Pre-AP Chemistry
About Pre-AP Chemistry
Teachers are strongly encouraged to take advantage of the full set of resources and
supports for Pre-AP Chemistry, which is summarized below. Some of these resources
must be used for a course to receive the Pre-AP Course Designation. To learn more
about the requirements for course designation, see details below and on page 69.
Included in this guide as well as in the Pre-AP Chemistry Teacher Resources, the
framework defines what students should know and be able to do by the end of the
course. It serves as an anchor for model lessons and assessments, and it is the primary
document teachers can use to align instruction to course content. Use of the course
framework is required. For more details see page 20.
Teacher resources, available in print and online, include a robust set of model lessons
that demonstrate how to translate the course framework, shared principles, and areas of
focus into daily instruction. Use of the model lessons is encouraged but not required.
For more details see page 42.
Accessed through Pre-AP Classroom (the Pre-AP digital platform), these short
formative assessments provide insight into student progress. They are automatically
scored and include multiple-choice and technology-enhanced items with rationales
that explain correct and incorrect answers. Use of one learning checkpoint per unit is
required. For more details see page 44.
Available in the printed teacher resources as well as on Pre-AP Classroom,
performance tasks allow students to demonstrate their learning through extended
problem-solving, writing, analysis, and/or reasoning tasks. Scoring guidelines are
provided to inform teacher scoring, with additional practice and feedback suggestions
available in online modules on Pre-AP Classroom. Use of each unit’s performance
task is required. For more details see page 46.
Available in the student resources, with supporting materials in the teacher resources, these
tasks provide an opportunity for students to practice applying skills and knowledge as they
would in a performance task, but in a more scaffolded environment. Use of the practice
performance tasks is encouraged but not required. For more details see page 47.

17
Introduction to Pre-AP Chemistry
About Pre-AP Chemistry
Accessed through Pre-AP Classroom, the final exam serves as a classroom-based,
summative assessment designed to measure students’ success in learning and applying
the knowledge and skills articulated in the course framework. Administration of the
final exam is encouraged but not required. For more details see page 63.
Both the four-day Pre-AP Summer Institute (Pre-APSI) and the Online Foundational
Module Series support teachers in preparing and planning to teach their Pre-AP
course. All Pre-AP teachers are required to either attend the Pre-AP Summer
Institute or complete the module series. In addition, teachers are required to
complete at least one Online Performance Task Scoring module. For more details see
page 9.

Course Map
The course map shows how components are positioned throughout
the course. As the map indicates, the course is designed to be taught
over 140 class periods (based on 45-minute class periods), for a total
of 28 weeks.
Model lessons are included for approximately 50% of the total
instructional time, with the percentage varying by unit. Each unit is
divided into key concepts.
TEACH
The model lessons demonstrate how the Pre-AP shared principles
and science areas of focus come to life in the classroom.
Shared Principles
Close observation and analysis
Higher-order questioning
Evidence-based writing
Academic conversation
Areas of Focus
Emphasis on analytical reading and writing
Strategic use of mathematics
Attention to modeling
Each unit includes two learning checkpoints and a performance task.
These formative assessments are designed to provide meaningful
feedback for both teachers and students.
The nal exam, available beginning in the 2021–22 school year,
is not represented in the map.
UNIT 1
Structure and
Properties of
Matter
~30 Class Periods
Pre-AP model lessons provided for
approximately 50% of instructional
time in this unit
Particle View of States of Matter
Learning Checkpoint 1
Phase Changes and Particle
Interactions
Kinetic Molecular Theory
Learning Checkpoint 2
Performance Task for Unit 1
Course Guide
© 2021 College Board
Pre-AP Chemistry
18

UNIT 2
Chemical
Bonding and
Interactions
~40 Class Periods
Pre-AP model lessons provided for
approximately 40% of instructional
time in this unit
Classication and Interactions of
Matter
Learning Objectives 2.2.A.1–2.2.C.1
Molecular Structure and Properties
Course Guide
© 2021 College Board
Pre-AP Chemistry
19
Learning Checkpoint 1
continued)
Learning Objectives 2.2.D.1–2.2.G.1
Molecular Structure and Properties
Covalent and Ionic Bonding
Learning Checkpoint 2
Performance Task for Unit 2
UNIT 3
Chemical
~30 Class Periods
Pre-AP model lessons provided for
approximately 30% of instructional
time in this unit
Counting Particles in Substances
Learning Checkpoint 1
Counting Particles in Chemical
Reactions
Learning Checkpoint 2
Performance Task for Unit 3
UNIT 4
Chemical
Transformations
~40 Class Periods
Pre-AP model lessons provided for
approximately 30% of instructional
time in this unit
Precipitation Chemistry
Oxidation–Reduction Chemistry
Learning Checkpoint 1
Acid–Base Chemistry
Thermochemistry
Reaction Rates
Learning Checkpoint 2
Performance Task for Unit 4

20
Course Guide
© 2021 College Board
Pre-AP Chemistry
About Pre-AP Chemistry
Pre-AP Chemistry Course Framework
Based on the Understanding by Design® (Wiggins and McTighe) model, the Pre-AP
Chemistry Course Framework is back mapped from AP expectations and aligned to
essential grade-level expectations. The course framework serves as a teacher’s blueprint
for the Pre-AP Chemistry instructional resources and assessments.
The course framework was designed to meet the following criteria:
Focused: The framework provides a deep focus on a limited number of concepts
and skills that have the broadest relevance for later high school, college, and career
success.
Measurable: The framework’s learning objectives are observable and measurable
statements about the knowledge and skills students should develop in the course.
Manageable: The framework is manageable for a full year of instruction, fosters
the ability to explore concepts in depth, and enables room for additional local or
state standards to be addressed where appropriate.
Accessible: The framework’s learning objectives are designed to provide all
students, across varying levels of readiness, with opportunities to learn, grow, and
succeed.

21
Pre-AP Chemistry Course Framework
About Pre-AP Chemistry
The Pre-AP Chemistry Course Framework includes the following components:
Big Ideas
The big ideas are recurring themes that allow students to create meaningful
connections between course concepts. Revisiting the big ideas throughout the
course and applying them in a variety of contexts allows students to develop deeper
conceptual understandings.
Enduring Understandings
Each unit focuses on a small set of enduring understandings. These are the long-term
takeaways related to the big ideas that leave a lasting impression on students. Students
build and earn these understandings over time by exploring and applying course
content throughout the year.
Key Concepts
To support teacher planning and instruction, each unit is organized by key concepts.
Each key concept includes relevant learning objectives and essential knowledge
statements and may also include content boundary
and cross connection statements.
These a
re illustrated and defined below.
Course Guide
© 2021 College Board
Pre-AP Chemistry
25
About Pre-AP Chemistry
Pre-AP Chemistry Course Framework
KEY CONCEPT 1.1: PARTICLE VIEW OF STATES OF MATTER
Analyzing how the macroscopic properties of solids, liquids, and gases can be explained by differences at the
particle level
Learning Objectives
Students will be able to ...
Essential Knowledge
Students need to know that ...
1.1.A.1 Create and/or evaluate models that illustrate
how the motion and arrangement of particles differ
among solids, liquids, and gases.
1.1.A.2 Describe how the properties of solids, liquids,
and gases are related to particle arrangement.
1.1.A.3 Create and/or evaluate models that illustrate
how changes in temperature influence the motion of
particles in solids, liquids, and gases.
1.1.A Properties of matter at the macroscopic level are related to
the particle structure of matter.
a. Solids, liquids, and gases have distinct macroscopic
properties, such as density and the ability to flow, that can
be understood qualitatively in terms of the arrangement of
particles and their degree of motion.
b. Particles of matter interact with one another and have the
ability to attract one another.
c. The kinetic energy of particles increases with temperature.
d. Mass is conserved during all physical and chemical particle
interactions.
1.1.B.1 Justify the choice of equipment used to make
a measurement, based on precision.
1.1.B.2 Record measured values to the proper
experimental precision.
1.1.B Recorded values must account for the precision of a
measurement.
a. The precision of a measurement is limited by the precision of
the instrument used to make the measurement.
b. Recorded values should include one estimated digit beyond
the scale of the instrument used to make the measurement.
1.1.C.1 Create and/or evaluate particulate and
graphical models representing the density of pure
substances.
1.1.C.2 Explain the relationship between the density
and the arrangement of particles within a pure
substance.
1.1.C.3 Perform calculations relating to the density of
pure substances.
1.1.C Density is a quantitative measure of the packing of particles
that make up matter.
a. The density of a substance is related to the mass of the
particles that make up that substance and to how tightly
these particles are packed.
b. The density of a substance can be represented by the slope
of the line on a graph that plots the mass of the substance
versus its volume.
c. The density of a gas is substantially lower than that of either
a solid or a liquid.
Content Boundary: This unit focuses on the properties and behavior of pure substances only. Mixtures are introduced in
Unit 2. The term particle is used throughout Unit 1. Differentiating between atoms and molecules is reserved for Unit 2.
Content Boundary: While error analysis is an essential component of laboratory work, significant figures are just one way
to account for limited precision. The application of the significant figure rules is not part of Pre-AP Chemistry.
Cross Connection: This unit builds on middle school knowledge that all matter is made up of particles. The focus of this
unit is on how the properties and behavior of those particles differ among the various states of matter and among different
types of matter.
Cross Connection: The use of scientific notation, the ability to convert units, and basic knowledge of the International
System of Units (SI) are considered prior knowledge.
CHEM_CG_CONF.indd 25 05/03/20 2:30 AM
Learning Objectives:
ese objectives
dene what a student
needs to be able to
do with essential
knowledge to progress
toward the enduring
understandings. e
learning objectives
serve as actionable
targets for instruction
and assessment.
Essential Knowledge
Statements:
e essential knowledge
statements are linked to one
or more learning objectives.
ese statements describe the
knowledge required to perform
the learning objective(s).
Content Boundary and Cross
Connection Statements:
When needed, content boundary
statements provide additional clarity
about the content and skills that lie within
versus outside of the scope of this course.
Cross connection statements highlight
important connections that should be
made between key concepts within
and across the units.

22
Course Guide
© 2021 College Board
Pre-AP Chemistry
Pre-AP Chemistry Course Framework
About Pre-AP Chemistry
While the Pre-AP Chemistry framework is organized into four core units of study,
the content is grounded in three big ideas, which are cross-cutting concepts that build
conceptual understanding and spiral throughout the course. Since these ideas cut
across units, they serve as the underlying foundation for the enduring understandings,
key concepts, learning objectives, and essential knowledge statements that make up the
focus of each unit.
The three big ideas that are central to deep and productive understanding in Pre-AP
Chemistry are:
Structure and Properties: All matter is composed of particles that are in
constant motion and interact with one another. This movement and interaction
is responsible for the observable properties of matter. Observed properties can be
used to infer the number and type(s) of particle(s) in a sample of matter.
Energy: Energy is transferred in all physical and chemical processes. During these
processes, energy is either redistributed within the system or between systems.
Transformations: At its heart, chemistry is about rearrangements of matter.
These rearrangements, or transformations, involve the breaking and forming of
intermolecular forces or chemical bonds. Macroscopic observations can be used to
quantify and describe these rearrangements at the atomic scale.

23
About Pre-AP Chemistry
Pre-AP Chemistry Course Framework
Unit 1: Structure and Properties of Matter
Solids, liquids, and gases have
different properties as a result of
the motion of particles and the
interactions among them.
All measurements have uncertainty,
and their level of precision must be
accounted for in the design of an
experiment and the recording of
data.
The amount of energy transferred
during heating and cooling matter or
changing its state is determined by
the interactions among the particles
that make up the matter.
Observable properties of gases can
be measured experimentally and
explained using an understanding of
particle motion.
Unit 2: Chemical Bonding and Interactions
The macroscopic physical properties
of materials can be explained by
the intermolecular forces among
particles.
The structure and properties of
compounds arise from the periodic
properties and bonding patterns of
the constituent atoms.
The mole concept is used to
quantitatively relate the number
of particles involved in a reaction
to experimental data about that
reaction.
In chemical reactions, bonding
between atoms changes, leading
to new compounds with different
properties.
Unit 4: Chemical Transformations
Solubility, electron transfer, and
proton transfer are driving forces in
chemical reactions.
All chemical reactions are
accompanied by a transfer of energy.
Chemical reactions occur at varying
rates that are related to the frequency
and success of collisions between
reactants.

24
Course Guide
© 2021 College Board
Pre-AP Chemistry
Pre-AP Chemistry Course Framework
About Pre-AP Chemistry
Unit 1: Structure and Properties of Matter
Suggested Timing: Approximately 6 weeks
This course progresses from macroscopic to atomic explorations of properties of
matter in order to help students develop a conceptual understanding of matter at the
molecular level. The first unit is designed to spark students’ interest in chemistry
as they make meaningful connections between the familiar world of everyday,
macroscopic variables and observations and the less familiar context of the motion and
interactions of particles at the atomic level.
By the end of this unit, students develop a set of simple rules to describe the behavior
of particles in pure substances through building and revising particulate models. They
deepen their understanding throughout the unit as they support and verify predictions
of these models using observations of real-world phenomena and calculations of various
physical properties such as the density of solids and liquids, the basic parameters of gases
such as pressure and volume, and the role energy plays in phase transitions. Students
also consider how the attraction among particles influences properties; the factors that
establish the strength of those forces will be explored in Unit 2.
Students will understand that ...
Solids, liquids, and gases have different properties as a result of the motion of
particles and the interactions among them.
All measurements have uncertainty, and their level of precision must be accounted
for in the design of an experiment and the recording of data.
The amount of energy transferred during heating and cooling matter or changing its
state is determined by the interactions among the particles that make up the matter.
Observable properties of gases can be measured experimentally and explained
using an understanding of particle motion.
1.1: Particle view of states of matter – Analyzing how the macroscopic properties
of solids, liquids, and gases can be explained by differences at the particle level
1.2: Phase changes and particle interactions – Examining the role energy plays
in phase transitions and how these transitions can be represented using phase
diagrams and heating curves
1.3: Kinetic molecular theory – Investigating gases and how their properties and
behavior can be predicted from the kinetic molecular theory

25
About Pre-AP Chemistry
Pre-AP Chemistry Course Framework
Analyzing how the macroscopic properties of solids, liquids, and gases can be explained by differences at the
particle level
Learning Objectives
Students will be able to ...
Essential Kno
wledge
Students need to know that ...
1.1.A.1 Create and/or evaluate models that illustrate
how the motion and arrangement of particles differ
among solids, liquids, and gases.
1.1.A.2 Describe how the properties of solids, liquids,
and gases are related to particle arrangement.
1.1.A.3 Create and/or evaluate models that illustrate
how changes in temperature influence the motion of
particles in solids, liquids, and gases.
1.1.A Properties of matter at the macroscopic level are related to
the particle structure of matter.
a. Solids, liquids, and gases have distinct macroscopic
properties, such as density and the ability to flow, that can
be understood qualitatively in terms of the arrangement of
particles and their degree of motion.
b. Particles of matter interact with one another and have the
ability to attract one another.
c. The kinetic energy of particles increases with temperature.
d. Mass is conserved during all physical and chemical particle
interactions.
1.1.B.1 Justify the choice of equipment used to make
a measurement, based on precision.
1.1.B.2 Record measured values to the proper
experimental precision.
1.1.B Recorded values must account for the precision of a
measurement.
a. The precision of a measurement is limited by the precision of
the instrument used to make the measurement.
b. Recorded values should include one estimated digit beyond
the scale of the instrument used to make the measurement.
1.1.C.1 Create and/or evaluate particulate and
graphical models representing the density of pure
substances.
1.1.C.2 Explain the relationship between the density
and the arrangement of particles within a pure
substance.
1.1.C.3 Perform calculations relating to the density of
pure substances.
1.1.C Density is a quantitative measure of the packing of particles
that make up matter.
a. The density of a substance is related to the mass of the
particles that make up that substance and to how tightly
these particles are packed.
b. The density of a substance can be represented by the slope
of the line on a graph that plots the mass of the substance
versus its volume.
c. The density of a gas is substantially lower than that of either
a solid or a liquid.
Content Boundary: This unit focuses on the properties and behavior of pure substances only. Mixtures are introduced in
Unit 2. The term particle is used throughout Unit 1. Differentiating between atoms and molecules is reserved for Unit 2.
Content Boundary: While error analysis is an essential component of laboratory work, significant figures are just one way
to account for limited precision. The application of the significant figure rules is not part of Pre-AP Chemistry.
Cross Connection: This unit builds on middle school knowledge that all matter is made up of particles. The focus of this
unit is on how the properties and behavior of those particles differ among the various states of matter and among different
types of matter.
Cross Connection: The use of scientific notation, the ability to convert units, and basic knowledge of the International
System of Units (SI) are considered prior knowledge.

26
Course Guide
© 2021 College Board
Pre-AP Chemistry
Pre-AP Chemistry Course Framework
About Pre-AP Chemistry
Examining the role energy plays in phase transitions and how these transitions can be represented
using phase diagrams and heating curves
Learning Objectives
Students will be able to …
Essential Knowledge
Students need to know that ...
1.2.A.1 Create and/or evaluate a claim about the
relationship between transfer of thermal energy and
the temperature change in different samples.
1.2.A.2 Perform calculations using data gathered from
a simple constant-pressure calorimetry experiment.
1.2.A The transfer of energy associated with a change in
temperature of a sample of matter is heat. Specific heat
capacity is a proportionality constant that relates the amount of
energy absorbed by a substance to its mass and its change in
temperature.
1.2.B.1 Use data to explain the direction of energy flow
into or out of a system.
1.2.B Energy transfers are classified as endothermic or
exothermic.
a. In endothermic changes, energy flows from the
surroundings to the system.
b. In exothermic changes, energy flows from the system to the
surroundings.
1.2.C.1 Explain the relationship between changes in
states of matter and the attractions among particles.
1.2.C.2 Create and/or interpret models representing
phase changes.
1.2.C Substances with stronger attractions among particles
generally have higher melting and boiling points than substances
with weaker attractions among particles.
1.2.D.1 Create and/or interpret heating and cooling
curves and/or phase diagrams of pure substances.
1.2.D.2 Calculate the energy transferred when a
substance changes state.
1.2.D The transitions between solid, liquid, and gas can be
represented with heating and cooling curves and phase
diagrams.
a. Heating and cooling curves represent how a substance
responds to the addition or removal of energy (as heat).
b. The temperature of a substance is constant during a phase
change.
c. Energy changes associated with a phase change can be
calculated using heat of vaporization or heat of fusion.
d. Phase diagrams give information about a pure substance
at a specific temperature and pressure, including phase
transitions.
Content Boundary: The study of critical points and triple points is beyond the scope of the course. The focus of the study
of phase diagrams should be on how the combination of temperature and pressure determine the state of matter of a given
substance and identification of phase changes.
Cross Connection: The study of energy transfer in Unit 1 is limited to physical changes. Students will revisit
thermochemistry in Unit 4, this time applied to chemical reactions.
Cross Connection: Forces of attraction between particles are identified as stronger or weaker in this unit as a way for
students to begin to understand differences in macroscopic properties of substances. Students will revisit these attractive
forces in Unit 2 as they learn about the types and relative strengths of intermolecular forces.

27
About Pre-AP Chemistry
Pre-AP Chemistry Course Framework
Investigating gases and how their properties and behavior can be predicted from the kinetic molecular
theory
Learning Objectives
Students will be able to ...
Essential Knowledge
Students need to know that ...
1.3.A.1 Create and/or evaluate models that illustrate
how a gas exerts pressure.
1.3.A.2 Explain the relationship between pressure in a
gas and collisions.
1.3.A The pressure of a gas is the force the gas applies to a unit
area of the container it is in.
a. Pressure arises from collisions of particles with the walls of
the container.
b. Pressure is measured using several different units that are
proportional to each other.
1.3.B.1 Explain the relationships between the
macroscopic properties of a sample of a gas using the
kinetic molecular theory.
1.3.B.2 Create and/or evaluate models that illustrate
how a sample of gas responds to changes in
macroscopic properties.
1.3.B The kinetic molecular theory relates the macroscopic
properties of a gas to the motion of the particles that comprise
the gas. An ideal gas is a gas that conforms to the kinetic
molecular theory.
1.3.C.1 Determine mathematically and/or graphically
the quantitative relationship between macroscopic
properties of gases.
1.3.C.2 Perform calculations relating to the
macroscopic properties of gases.
1.3.C The relationships between macroscopic properties of a
gas, including pressure, temperature, volume, and amount of gas,
can be quantified.
Content Boundary: All gases studied in this unit are considered to be ideal. The derivation and discussion of the ideal gas
law has been reserved for Unit 3, after students have been introduced to the mole.

28
Course Guide
© 2021 College Board
Pre-AP Chemistry
Pre-AP Chemistry Course Framework
About Pre-AP Chemistry
Unit 2: Chemical Bonding and Interactions
Suggested Timing: Approximately 8 weeks
This unit focuses on particle interactions and continues the unit progression from the
macroscopic to the atomic level. Building on prior concepts taught in middle school
about basic atomic structure, students build on and extend their understanding as
they explore how the shape and structure of particles—including atoms, molecules,
and ions—provide the explanatory framework for particle interactions. Students first
consider intermolecular forces and connect them to both macroscopic observations
and molecular structure. They then build on and deepen their preliminary
understanding of bonding concepts from middle school and should begin to
understand the electrostatic nature of many chemical interactions.
Throughout the unit, students revisit and revise the particulate models they developed
in Unit 1 to account for the role of particle interactions. The patterns found in the
periodic table are used to explain these phenomena.
Students will understand that ...
The macroscopic physical properties of materials can be explained by the
intermolecular forces among particles.
The structure and properties of compounds arise from the periodic properties and
bonding patterns of the constituent atoms.
2.1: Classification and interactions of matter – Describing and classifying matter,
with a focus on how intermolecular and intramolecular forces determine the
properties of matter
2.2: Molecular structure and properties – Relating the properties of molecular
compounds to molecular structure
2.3: Covalent and ionic bonding – Analyzing the differences between covalent and
ionic bonding, with an emphasis on the electrostatic nature of ionic attractions

29
About Pre-AP Chemistry
Pre-AP Chemistry Course Framework
Describing and classifying matter, with a focus on how intermolecular and intramolecular
forces determine the properties of matter
Learning Objectives
Students will be able to ...
Essential Knowledge
Students need to know that ...
2.1.A.1 Distinguish between atoms, molecules, and
compounds at the particle level.
2.1.A.2 Create and/or evaluate models of pure
substances.
2.1.A A pure substance always has the same composition. Pure
substances include elements, molecules, and compounds.
a. An element is composed of only one type of atom.
b. A molecule is a particle composed of more than one atom.
c. A compound is composed of two or more elements and has
properties distinct from those of its component atoms.
2.1.B.1 Create and/or evaluate models of mixtures.
2.1.B.2 Interpret the results of an experiment involving
the separation of a mixture.
2.1.B A mixture is composed of two or more different types of
particles that are not bonded.
a. Each component of a mixture retains its unique properties.
b. Mixtures can be separated using physical processes such as
filtration, evaporation, distillation, and chromatography.
2.1.C.1 Relate the total and partial pressure of a
gas mixture to the number of particles and their
proportions.
2.1.C In a mixture of gases, each gas contributes to the pressure
of the gas.
a. The total pressure of the mixture is the sum of the individual
partial pressures of each gas that makes up the mixture.
b. The partial pressures of each gas can be determined by
comparing the fraction of particles of the gas in the mixture
to the total number of gas particles.
2.1.D.1 Create and/or evaluate a claim about the types
of forces that are overcome during the melting, boiling,
and/or dissolving of substances.
2.1.D Attractions among particles of matter are the result of
electrostatic interactions between particles.
a. Intermolecular forces are responsible for many physical
properties of substances including boiling point, melting
point, surface tension, and volatility.
b. Intramolecular forces hold atoms together in a molecule.
Cross Connection: Unit 1 treats particles as if they have no internal structure and are mostly identical. In this unit, students
begin to distinguish between atoms and molecules and between mixtures and pure substances.
Cross Connection: The basics of atomic structure, including the shell model of the atom and the properties of the three
basic subatomic particles, are considered prior knowledge from middle school.

30
Course Guide
© 2021 College Board
Pre-AP Chemistry
Pre-AP Chemistry Course Framework
About Pre-AP Chemistry
Relating the properties of molecular compounds to molecular structure
Learning Objectives
Students will be able to ...
Essential Knowledge
Students need to know that ...
2.2.A.1 Create and/or evaluate models that illustrate
how molecular properties influence the type(s) of
intermolecular force(s) present in a substance.
2.2.A.2 Create and/or evaluate a claim about the
type(s), strength(s), and origin(s) of intermolecular
forces present in a substance.
2.2.A Intermolecular forces occur between molecules and are
the result of electrostatic interactions.
a. London dispersion forces are attractions among temporary
dipoles created by the random movement of electrons;
these attractions occur between all types of molecules.
Molecules with more electrons tend to have stronger
London dispersion forces.
b. Dipole–dipole forces are attractions among permanent
dipoles on interacting molecules.
c. Hydrogen bonding forces exist when hydrogen atoms
covalently bonded to highly electronegative atoms (N, O, or
F) are attracted to the negative ends of dipoles formed by
highly electronegative atoms (N, O, or F) in other molecules.
2.2.B.1 Create and/or evaluate a claim that uses
relative strength of intermolecular forces to explain
trends in the physical properties of substances.
2.2.B Intermolecular forces can be used to explain trends in
physical properties of substances including boiling point, melting
point, surface tension, volatility, and solubility.
2.2.C.1 Describe trends in properties of elements
based on their position in the periodic table and the
shell model of the atom.
2.2.C The periodic table is an organizational tool for elements
based on their properties.
a. Patterns of behavior of elements are based on the number
of electrons in the outermost shell (valence electrons).
b. Important periodic trends include electronegativity and
atomic radius.
2.2.D.1 Create and/or evaluate Lewis diagrams for
molecular compounds and/or polyatomic ions.
2.2.D.2 Determine if given molecules are structural
isomers.
2.2.D A Lewis diagram is a simplified representation of a
molecule.
a. Lewis diagrams show the bonding patterns between atoms
in a molecule.
b. Molecules with the same number and type of atoms but
different bonding patterns are structural isomers, which
have different properties from one another.
2.2.E.1 Determine molecular geometry from a Lewis
diagram using valence shell electron pair repulsion
theory.
2.2.E Valence shell electron pair repulsion (VSEPR) theory
predicts molecular geometry from a Lewis diagram. Molecular
geometries include linear, bent, trigonal planar, trigonal
pyramidal, and tetrahedral arrangements of atoms.
2.2.F.1 Determine the polarity of a molecule from its
molecular geometry and electron distribution.
2.2.F Molecules with asymmetric distributions of electrons are
polar.
2.2.G.1 Create and/or evaluate a claim about the
strength and type(s) of intermolecular forces present
in a sample based on molecular polarity.
2.2.G Molecular geometry determines if a molecule has a
permanent dipole and therefore the type(s) of intermolecular
forces present in that molecule.

31
About Pre-AP Chemistry
Pre-AP Chemistry Course Framework
Content Boundary: The study of expanded octets, resonance structures, and formal charge is beyond the scope of
this course. Rather than focusing on exceptions to the octet rule, the focus is on helping students develop a deep
understanding of the rationale for molecular structure. If students go on to take AP Chemistry, this introduction will provide
the foundation for more advanced study.
Content Boundary: The quantum mechanical model of the atom and the writing of electron configurations are beyond the
scope of this course. If students go on to take AP Chemistry, they will study the details of the electron structure of atoms,
including electron configurations.
Content Boundary: The study of isomers is limited to structural isomers and is included so students can begin to develop
an understanding that in addition to the number and type of atoms in a molecule, the arrangement of the atoms and bonds
is also important in determining properties.
Cross Connection: Students should connect their study of phase changes and properties of matter from Unit 1 to
intermolecular forces. This key concept leads with the study of intermolecular forces rather than building up to it. This
approach enables students to immediately begin connecting macroscopic observations to atomic-level understandings
even while they are learning about Lewis structures and molecular geometry. If students go on to take AP Chemistry, they
will continue to build on their understanding of intermolecular forces.

32
Course Guide
© 2021 College Board
Pre-AP Chemistry
Pre-AP Chemistry Course Framework
About Pre-AP Chemistry
Analyzing the differences between covalent and ionic bonding, with an emphasis on the electrostatic
nature of ionic attractions
Learning Objectives
Students will be able to ...
Essential Knowledge
Students need to know that ...
2.3.A.1 Create and/or evaluate a claim about the type
of bonding in a compound based on its component
elements and its macroscopic properties.
2.3.A Bonding between elements can be nonpolar covalent, polar
covalent, or ionic.
2.3.B.1 Interpret the results of an experiment to
determine the type of bonding present in a substance.
2.3.B Ionic and covalent compounds have different properties
based on their bonding.
a. Properties of ionic compounds result from electrostatic
attractions of constituent ions.
b. Properties of covalent compounds result from bonds
created by the sharing of electrons and intermolecular
forces.
2.3.C.1 Explain the relationship between the relative
strength of attractions between cations and anions in
an ionic solid in terms of the charges of the ions and
the distance between them.
2.3.C Ionic solids are made of cations and anions.
a. The relative number of cations and anions retain overall
electrical neutrality.
b. As the charge on each ion increases the relative strength of
the interaction will also increase.
c. As the distance between ions increases the relative strength
of the interaction will decrease.
2.3.D.1 Create and/or evaluate representations of ionic
and covalent compounds.
2.3.D Ionic and covalent compounds can be represented by
particulate models, structural formulas, chemical formulas, and
chemical nomenclature.
Content Boundary: The study of ionic compounds should include those compounds containing the polyatomic ions listed
on the Pre-AP Chemistry equation sheet. The naming of acids and organic compounds is beyond the scope of this course.
Nomenclature should be consistent with recommendations of the International Union of Pure and Applied Chemistry
(IUPAC).
Content Boundary: While students should have a conceptual understanding of the role electrostatic interactions play in
ionic compounds, quantitative applications of Coulomb’s law are beyond the scope of this course. If students go on to take
AP Chemistry or AP Physics, they will study Coulomb’s law in more detail.

33
About Pre-AP Chemistry
Pre-AP Chemistry Course Framework
Suggested Timing:Approximately 6 weeks
This unit explores chemical transformations of matter by building on the physical
transformations studied in Units 1 and 2. Leveraging what has been learned about
particles in Units 1 and 2, this unit introduces students to the importance of the mole
concept for collecting data about particles and chemical reactions. Since chemistry
deals with large numbers of particles, students are introduced to the idea of counting
by weighing. To reinforce the particle nature of matter studied in Units 1 and 2,
students use particulate representations of reactions to connect the amount of reactant
consumed and the amount of product formed to the rearrangement of particles on the
molecular level. Students will also use balanced chemical equations and mathematics to
reason about amounts of reactants and products in chemical reactions.
Students will understand that ...
The mole concept is used to quantitatively relate the number of particles involved
in a reaction to experimental data about that reaction.
In chemical reactions, bonding between atoms changes, leading to new compounds
with different properties.
3.1: Counting particles in substances – Using the mole concept to count by
weighing
3.2: Counting particles in chemical reactions – Reasoning about amounts of
reactants and products in chemical reactions using balanced chemical equations

34
Course Guide
© 2021 College Board
Pre-AP Chemistry
Pre-AP Chemistry Course Framework
About Pre-AP Chemistry
Using the mole concept to count by weighing
Learning Objectives
Students will be able to ...
Essential Knowledge
Students need to know that ...
3.1.A.1 Explain the relationship between the mass of a
substance, the number of particles of that substance,
and the number of moles of that substance.
3.1.A.2 Use the mole concept to calculate the mass,
number of particles, or number of moles of a given
substance.
3.1.A A large number of particles of a substance is needed to
measure the physical properties of that substance.
a. A mole of a substance contains Avogadro’s number
(6.0
21
0)
23
×
of particles.
b. The molar mass of an element listed on the periodic table is
the mass, in grams, of a mole of atoms of that element.
3.1.B.1 Explain the relationships between
macroscopic properties of gas samples.
3.1.B.2 Perform calculations using the ideal gas law.
3.1.B.3 Create and/or evaluate models based on the
ideal gas law.
3.1.B The ideal gas law describes the mathematical relationship
between pressure, volume, number of gas particles, and
temperature.
a. Two samples of gas with the same pressure, volume, and
temperature have the same number of particles.
b. The mass of the particles can be computed from atomic
masses.
c. Because macroscopic samples of a gas contain many
particles, moles are useful units for counting particles.
Content Boundary: The determination of empirical and molecular formulas is beyond the scope of this course.
Cross Connection: The focus on gases in this key concept about the mole allows students to draw connections between
this unit and what they learned about gases in Units 1 and 2. Gases are a useful context for learning about the mole
because a large quantity of gas is needed to measure properties of the gas.
35
About Pre-AP Chemistry
Pre-AP Chemistry Course Framework
Reasoning about amounts of reactants and products in chemical reactions using balanced chemical
equations
Learning Objectives
Students will be able to ...
Essential Knowledge
Students need to know that ...
3.2.A.1 Create and/or evaluate models of chemical
transformations.
3.2.A All chemical transformations involve the rearrangement of
atoms to form new combinations.
a. Since the atoms are not created or destroyed, the total
numbers of each atom must remain constant.
b. Chemical transformations can be modeled by balanced
chemical equations and particulate representations.
3.2.B.1 Explain the relationship between the quantity
of reactants consumed and the quantity of products
formed in a chemical transformation.
3.2.B.2 Perform stoichiometric calculations involving
the quantity of reactants and products in a chemical
system.
3.2.B A balanced chemical reaction equation, combined with
the mole concept, can be used to quantify the amounts of
reactants consumed and products formed during a chemical
transformation.
3.2.C.1 Create and/or evaluate models of a reaction
mixture before and/or after a reaction has occurred,
including situations with a limiting reactant.
3.2.C The limiting reactant is the reactant that is completely
consumed during a chemical reaction. The limiting reactant
determines the amount of product formed.
3.2.D.1 Calculate the theoretical yield and/or percent
yield of a chemical reaction.
3.2.D A balanced chemical reaction equation, combined with
the mole concept, can be used to calculate the theoretical and
percent yield of a reaction.
Content Boundary: Stoichiometric calculations involving limiting reactants are limited to whole numbers of moles (for
both the initial and final quantities), such as what could be represented in particle diagrams to focus on conceptual
understanding instead of algorithmic calculations.
Cross Connection: Stoichiometric calculations will be used in Unit 4 to investigate specific types of reactions.

36
Course Guide
© 2021 College Board
Pre-AP Chemistry
Pre-AP Chemistry Course Framework
About Pre-AP Chemistry
Unit 4: Chemical Transformations
Suggested Timing: Approximately 8 weeks
In this unit, students explore the primary driving forces in chemical reactions through
symbolic, particulate, and mathematical representations. The study of precipitation
reactions, oxidation–reduction reactions, and acid–base reactions allows students to
apply what they have learned about bonding in Unit 2 and stoichiometric relationships
in Unit 3 as they explore specific reaction types and predict products of reactions.
An emphasis on net-ionic equations allows students to focus on the substances that
are directly involved in chemical reactions. Students will also revisit and extend the
concepts of energy from Unit 1 as they apply them to energy changes involved in
chemical transformations, building to the fundamental understanding that breaking
chemical bonds requires energy and that bond formation releases energy. Students will
also study the rates of chemical reactions and factors that influence the rates, using a
particulate perspective.
Students will understand that …
Solubility, electron transfer, and proton transfer are driving forces in chemical
reactions.
All chemical reactions are accompanied by a transfer of energy.
Chemical reactions occur at varying rates that are related to the frequency and
success of collisions between reactants.
4.1: Precipitation chemistry – Investigating how solubility is related to
precipitation and can drive chemical reactions
4.2: Oxidation–reduction chemistry – Analyzing how electron transfer can drive
chemical reactions
4.3: Acid–base chemistry – Examining properties of acids and bases and how
proton transfer can drive chemical reactions
4.4: Thermochemistry – Extending the study of energy by analyzing energy
transformations that occur during chemical reactions
4.5: Reaction rates – Investigating the factors that influence reaction rates

37
About Pre-AP Chemistry
Pre-AP Chemistry Course Framework
Investigating how solubility is related to precipitation and can drive chemical reactions
Learning Objectives
Students will be able to ...
Essential Knowledge
Students need to know that ...
4.1.A.1 Predict the products of a precipitation
reaction.
4.1.A Precipitation reactions may occur when two aqueous
solutions are mixed, because some ionic compounds are
insoluble in water and therefore precipitate out of solution.
4.1.B.1 Create and/or evaluate models of precipitation
reactions.
4.1.B Precipitation reactions can be modeled by molecular
equations, net ionic equations, and particulate representations.
4.1.C.1 Create and/or evaluate models that represent
the concentration of a solution.
4.1.C.2 Perform calculations relating to the molarity of
solutions.
4.1.C Molarity is one way to quantify the concentration of a
solution. It describes the number of dissolved particles in a unit
volume of that solution.
4.1.D.1 Predict the amount of solid produced in a
precipitation reaction using gravimetric analysis based
on the concentrations of the starting solutions.
4.1.D.2 Evaluate the results of a gravimetric analysis.
4.1.D Gravimetric analysis is a quantitative method for
determining the amount of a substance by selectively
precipitating the substance from an aqueous solution.
Content Boundary: The focus of predicting products of precipitation reactions is not to have students memorize solubility
rules or use a table of solubilities. Instead, students should focus on understanding that all sodium, potassium, ammonium,
and nitrate salts are soluble in water.
Cross Connection: Students continue to use principles of stoichiometry learned in Unit 3, now applied to precipitation
reactions.

38
Course Guide
© 2021 College Board
Pre-AP Chemistry
Pre-AP Chemistry Course Framework
About Pre-AP Chemistry
Analyzing how electron transfer can drive chemical reactions
Learning Objectives
Students will be able to ...
Essential Knowledge
Students need to know that ...
4.2.A.1 Identify a reaction as an oxidation–reduction
reaction based on the change in oxidation numbers of
reacting substances.
4.2.A.2 Create and/or evaluate a claim about which
reacting species is oxidized or reduced in an
oxidation–reduction reaction.
4.2.A Electrons are transferred between reactants in oxidation–
reduction (redox) reactions.
a. Substances lose electrons in the process of oxidation and
gain electrons in the process of reduction.
b. Oxidation numbers are useful for determining if electrons
are transferred in a chemical reaction.
c. Electrons are conserved in redox reactions.
4.2.B.1 Predict whether a redox reaction will occur
between two reactants using an activity series.
4.2.B.2 Create and/or evaluate an activity series from
experimental measurements.
4.2.B An activity series lists elements in order of decreasing
ease of oxidation and can be used to determine whether a redox
reaction will occur between two species.
4.2.C.1 Create and/or evaluate models of redox
reactions.
4.2.C Redox reactions can be modeled by molecular equations,
net ionic equations, and particulate representations.
Content Boundary: Oxidation–reduction is a broad classification of reactions, including synthesis, decomposition, and
combustion reactions. However, predicting products for oxidation–reduction reactions is limited to single-replacement
reactions.
Cross Connection: Students continue to use principles of stoichiometry learned in Unit 3, now applied to oxidation–
reduction reactions.

39
About Pre-AP Chemistry
Pre-AP Chemistry Course Framework
Examining properties of acids and bases and how proton transfer can drive chemical reactions
Learning Objectives
Students will be able to ...
Essential Knowledge
Students need to know that ...
4.3.A.1 Create and/or evaluate models of strong and
weak acids and bases.
4.3.A.2 Distinguish between strong and weak acids in
terms of degree of dissociation in aqueous solution.
4.3.A.3 Evaluate a claim about whether a compound is
a strong or weak acid or base.
4.3.A Acids and bases are described as either strong or weak
based on the degree to which they dissociate in aqueous
solution.
4.3.B.1 Explain the relationship between the hydrogen
ion concentration and the pH of a solution.
4.3.B.2 Calculate the pH of a solution.
4.3.B The pH of a solution is a measure of the molarity of H
3
O
+
(or
H
+
) in the solution.
4.3.C.1 Predict the products of a reaction between a
strong acid and a strong base.
4.3.C Acid–base reactions involve the transfer of a hydrogen ion
from the acid to the base. Strong acid–base reactions produce
water and an aqueous ionic compound.
4.3.D.1 Create and/or evaluate models of a reaction
between a strong acid and a strong base.
4.3.D Acid–base reactions can be modeled by molecular
equations, net ionic equations, and particulate representations.
Content Boundary: The study of acids and bases is limited to the Arrhenius and Brønsted-Lowry definitions. According to
these definitions, strong acids include HCl, HBr, HI, H
2
SO
4
, HClO
4
, and HNO
3
, and strong bases include group 1 and group 2
metal hydroxides (e.g., NaOH and KOH).
Cross Connection: Students continue to use principles of stoichiometry learned in Unit 3, now applied to acid–base
reactions.

40
Course Guide
© 2021 College Board
Pre-AP Chemistry
Pre-AP Chemistry Course Framework
About Pre-AP Chemistry
Extending the study of energy by analyzing energy transformations that occur during chemical
reactions
Learning Objectives
Students will be able to ...
Essential Knowledge
Students need to know that ...
4.4.A.1 Create and/or evaluate a claim about whether
a reaction is endothermic or exothermic from
experimental observations.
4.4.A.2 Explain the relationship between the measured
change in temperature of a solution and the energy
transferred by a chemical reaction.
4.4.A.3 Calculate energy changes in chemical
reactions from calorimetry data.
4.4.A A temperature change during a reaction is the result of
energy transfer during the process of breaking and forming
bonds.
a. Bond breaking is always an endothermic process and bond
formation is always an exothermic process.
b. Calorimetry can be used to quantify energy changes in a
reaction.
4.4.B.1 Create and/or evaluate a claim about the
energy transferred as a result of a chemical reaction
based on bond energies.
4.4.B The relative strength of bonds in reactants and products
determines the energy change in a reaction. Bond energy tables
and Lewis diagrams provide a way to estimate these changes
quantitatively for a wide variety of chemical reactions.
Content Boundary: The focus of the study of bond energy should be on the fundamental understanding that bond
breaking requires energy and bond formation releases energy rather than on algorithmic calculations.
Cross Connection: Students apply their knowledge of molecular structure from Unit 2 in the study of bond energy.

41
About Pre-AP Chemistry
Pre-AP Chemistry Course Framework
Investigating the factors that influence reaction rates
Learning Objectives
Students will be able to ...
Essential Knowledge
Students need to know that ...
4.5.A.1 Construct and/or evaluate particulate
representations that illustrate how changes in
concentration, temperature, or surface area of
reactants alter the rate of a chemical reaction.
4.5.A.2 Explain how experimental changes in the
rate of a reaction are related to changes in the
concentration, temperature, or surface area of the
reactants.
4.5.A The rate of a chemical reaction can be measured by
determining how quickly reactants are transformed into
products.
a. The reaction rate is related to the frequency of collisions
between reactant species and the proportion of effective
collisions.
b. The frequency of collisions increases with the concentration
of gases or dissolved species and with the surface area of a
solid.
c. The proportion of effective collisions increases directly as
temperature increases.
Content Boundary: The study of rate laws and mechanisms is beyond the scope of the course. If students go on to take AP
Chemistry, they will study kinetics in much more depth.
Cross Connection: The study of reaction rates relies on an understanding of the particle nature of matter that has been
developed in Units 1 through 3.

42
Course Guide
© 2021 College Board
Pre-AP Chemistry
About Pre-AP Chemistry
Pre-AP Chemistry Model Lessons
Model lessons in Pre-AP Chemistry are developed in collaboration with chemistry
educators across the country and are rooted in the course framework, shared
principles, and areas of focus. Model lessons are carefully designed to illustrate on-
grade-level instruction. Pre-AP strongly encourages teachers to internalize the lessons
and then offer the supports, extensions, and adaptations necessary to help all students
achieve the lesson goals.
The purpose of these model lessons is twofold:
Robust instructional support for teachers: Pre-AP Chemistry model lessons are
comprehensive lesson plans that, along with accompanying student resources,
embody the Pre-AP approach to teaching and learning. Model lessons provide clear
and substantial instructional guidance to support teachers as they engage students
in the shared principles and areas of focus.
Key instructional strategies: Commentary and analysis embedded in each lesson
highlights not just what students and teachers do in the lesson, but also how and
why they do it. This educative approach provides a way for teachers to gain unique
insight into key instructional moves that are powerfully aligned with the Pre-AP
approach to teaching and learning. In this way, each model lesson works to support
teachers in the moment of use with students in their classroom.
Teachers have the option to use any or all model lessons alongside their own locally
developed instructional resources. Model lessons target content areas that tend to be
challenging for teachers and students. While the lessons are distributed throughout
all four units, they are concentrated more heavily in the beginning of the course
to support teachers and students in establishing a strong foundation in the Pre-AP
approach to teaching and learning.

43
About Pre-AP Chemistry
Pre-AP Chemistry Model Lessons
The following support features recur throughout the Pre-AP Chemistry lessons, to
promote teacher understanding of the lesson design and provide direct-to-teacher
strategies for adapting lessons to meet their students’ needs:
Instructional Rationale
Guiding Student Thinking
Meeting Learners’ Needs
Classroom Ideas
Teacher Resource
© 2021 College Board
23
TEACH
Key Concept 2.1: Classication and Interactions of Matter
Pre-AP Chemistry
UNIT 2
Lesson 2.2: Atoms, Molecules, and Particles
Ask them to pair up each of these cards with one
of the cards they worked with previously (the “all
atoms shown” cards: A1, A4, and A7). As you
circulate around the room, guide students to the
intended associations.
The pairs are A1 and A9, A4 and A3, and
A7 and A2.
Once students have made their connections, give
each group the set of three cards showing both
representations (A6, A8, and A5).
Guiding Student Thinking
By introducing this mathematical notation aer students have done these calculations
in their own way, you can help them connect the above mathematical form to their
approach. Consider asking them if this mathematical form makes sense to them
and if they think it makes it easier to nd the number of atoms from the number
of molecules. Ratios such as 14 atoms/molecule are important in many chemical
computations and this provides an early exposure in a context that may be easier to
grasp than later applications (e.g., use of grams/mol).
Next, give each group the three “molecules as single particles” cards for butane (A9,
A3, and A2).
Handout 2.2
Unit 2: Chemical Bonding and Interactions
Student Resource
© 2021 College Board
Pre-AP Chemistry
11
HANDOUT
2.2
A7
A9
A8
Lesson 2.2: Atoms, Molecules, and Particles
CHEM_U2_SR_CONF.indd 11 29/01/20 6:42 PM
Unit 2: Chemical Bonding and Interactions
Student Resource
© 2021 College Board
Pre-AP Chemistry
9
Lesson 2.2: Atoms, Molecules, and Particles
Atoms, Molecules, and Particles – Card Sets
Card Set A
HANDOUT
2.2
A1
A3
A5
A2
A4
A6
CHEM_U2_SR_CONF.indd 9 29/01/20 6:42 PM
Unit 2: Chemical Bonding and Interactions
Student Resource
© 2021 College Board
Pre-AP Chemistry
9
Lesson 2.2: Atoms, Molecules, and Particles
Atoms, Molecules, and Particles – Card Sets
Card Set A
HANDOUT
2.2
A1
A3
A5
A2
A4
A6
CHEM_U2_SR_CONF.indd 9 29/01/20 6:42 PM
Meeting Learners’ Needs
If students are having
trouble drawing
connections between the
two sets of cards, you
can go ahead and give
them the cards showing
both representations,
used in the next step.
ese can provide extra
support as students are
working on the pairings.
CHEM_U2_TR_CONF.indd 23 29/01/20 6:46 PM
Teacher Resource
© 2021 College Board
9
TEACH
Pre-AP Chemistry
UNIT 4
Lesson 4.7: Bond Energy and Fuel Reactions
Key Concept 4.4: Thermochemistry
Lesson 4.7: Bond Energy and Fuel Reactions
Unit 4: Chemical Transformations
Student Resource
© 2021 College Board
Pre-AP Chemistry
7
HANDOUT
4.7.C
Modeling Bond Energy
Use the components from Handout 4.7.B to model breaking and forming bonds in
reactions to determine the temperature change during the reaction. The bond strengths
are shown in the following table.
Reaction Bond Strength
+
+
+
100 kJ/mol
200 kJ/mol
300 kJ/mol
+
+
+
100 kJ/mol
200 kJ/mol
300 kJ/mol
+
+
+
100 kJ/mol
200 kJ/mol
300 kJ/mol
+
+
+
100 kJ/mol
200 kJ/mol
300 kJ/mol
+
+
+
100 kJ/mol
200 kJ/mol
300 kJ/mol
+
+
+
100 kJ/mol
200 kJ/mol
300 kJ/mol
1. Consider the following reaction:
++
(a) What type of reaction is this?
Type 1: A chemical reaction that breaks strong bonds and forms
weak bonds.
Type 2: A chemical reaction that breaks weak bonds and forms
strong bonds.
Explain your thinking.
(b) Use the calorimeter representation on Handout 4.7.B to model this reaction
and record your final temperature here:
Initial temperature = 25°C Final temperature =
CHEM_U4_L7_SR_3R.indd 7 30/01/20 11:29 AM
Handout 4.7.C
Instructional Rationale
is part of the lesson is designed to give students a strong conceptual understanding
of the energetics of the breaking and forming of bonds before they do mathematical
calculations.
Begin the demonstration for the class by using
the following steps to model breaking a bond in
the red-red diatomic molecule shown in Handout
4.7.C. As you work through the demonstration,
be sure to highlight for students how to use the
various components since they will use them in
Part 3 of the lesson. A diagram of the setup is
shown on the following page.
1. Use four red bingo chips and the “+” and “→” cutouts to represent breaking a
bond in the “Reaction being modeled” area.
2. Place four red bingo chips, arranged as two diatomic molecules, in the
“Molecular-level view” box.
3. Place 100 kJ markers on the thermometer up to 25°C.
4. Put the initial temperature marker next to the thermometer at 25°C.
Classroom Ideas
You can show your
manipulations using
a document camera
so all students can see
the demonstration.
CHEM_U4_L7_TR_3R.indd 9 30/01/20 8:06 PM
Optional dierentiation strategies
to address diverse learning needs,
such as ideas for just-in-time skill
building during a lesson or ways to
break a task into smaller tasks, if
needed, to make it more accessible.
Classroom Ideas:
Tips related to the logistics
of the instruction, such as
suggestions for alternative
laboratory materials, or ways
to alleviate pacing concerns.
Guiding Student
Thinking:
Ways to facilitate
productive student
thinking and prevent
or address student
misconceptions in critical
areas of the lesson.
Instructional Rationale:
Insight into the strategic
design and purpose of
the instructional choices,
ow, and scaolding
within the model lesson.
Rationales oen describe
how a concept is continued
later in the lesson or unit.

44
Course Guide
© 2021 College Board
Pre-AP Chemistry
About Pre-AP Chemistry
Pre-AP Chemistry Assessments for Learning
Pre-AP Chemistry assessments function as a component of the teaching and learning
cycle. Progress is not measured by performance on any single assessment. Rather,
Pre-AP Chemistry offers a place to practice, to grow, and to recognize that learning
takes time. The assessments are updated and refreshed periodically.
Based on the Pre-AP Chemistry Course Framework, the learning checkpoints
require students to examine data, models, diagrams, and short texts—set in authentic
contexts—and to use quantitative reasoning in order to respond to a targeted set of
questions that measure students’ application of the key concepts and skills from the
unit. All eight learning checkpoints are automatically scored, with results provided
through feedback reports that contain explanations of all questions and answers as well
as individual and class views for educators. Teachers also have access to assessment
summaries on Pre-AP Classroom, which provide more insight into the question sets
and targeted learning objectives for each assessment event.
The following tables provide a synopsis of key elements of the Pre-AP Chemistry
learning checkpoints.
Format
Two learning checkpoints per unit
Digitally administered with automated scoring and
reporting
Questions target both concepts and skills from the course
framework
Time Allocated
Designed for one 45-minute class period per assessment
11–14 questions per assessment
9–12 four-option multiple choice
2–5 technology-enhanced questions

45
Pre-AP Chemistry Assessments for Learning
About Pre-AP Chemistry
Domains Assessed
Key Concepts
Key concepts and prioritized learning objectives from the
course framework
Skills
Three skill categories aligned to the Pre-AP science areas
of focus are assessed regularly across all eight learning
checkpoints:
emphasis on analytical reading and writing
strategic use of mathematics
attention to modeling
Question sets consist of two to three questions that focus
on a single stimulus or group of related stimuli, such as
texts, graphs, or tables.
Questions are set in authentic chemistry contexts.
Please see page 64 for a sample question set that illustrates
the types of questions included in Pre-AP learning
checkpoints and the Pre-AP final exam.

46
Course Guide
© 2021 College Board
Pre-AP Chemistry
Pre-AP Chemistry Assessments for Learning
About Pre-AP Chemistry
Each unit includes one performance-based assessment designed to evaluate the depth
of student understanding of key concepts and skills that are not easily assessed in a
multiple-choice format.
Some performance tasks mirror the AP free-response question style. Others engage
students in hands-on data collection and analysis in the laboratory. Students
demonstrate their understanding of content by analyzing scientific texts, data, and
models in order to develop analytical written responses to open-ended questions.
Students also use mathematics to support their chemical reasoning.
The performance tasks give students an opportunity to closely observe and analyze
real-world chemistry scenarios and apply the skills and concepts from across the course
units.
These tasks, developed for high school students across a broad range of readiness
levels, are accessible while still providing sufficient challenge and the opportunity to
practice the analytical skills that will be required in AP science courses and for college
and career readiness. Teachers participating in the official Pre-AP Program will receive
access to online learning modules to support them in evaluating student work for each
performance task.
Format
One performance task per unit
Administered in print
Educator scored using scoring guidelines
Time Allocated
Approximately 45 minutes or as indicated
An open-response task with multiple parts
Domains Assessed
Key Concepts
Key concepts and prioritized learning objectives from
the course framework
Skills
Three skill categories aligned to the Pre-AP science
areas of focus:
emphasis on analytical reading and writing
strategic use of mathematics
attention to modeling

47
Pre-AP Chemistry Assessments for Learning
About Pre-AP Chemistry
A practice performance task in each unit provides students with the opportunity to
practice applying skills and knowledge in a context similar to a performance task,
but in a more scaffolded environment. These tasks include strategies for adapting
instruction based on student performance and ideas for modifying or extending tasks
based on students’ needs.

48
Course Guide
© 2021 College Board
Pre-AP Chemistry
Pre-AP Chemistry Assessments for Learning
About Pre-AP Chemistry
The following task and set of scoring guidelines are representative of what students and
educators will encounter on the performance tasks. (The example below is a practice
performance task from Unit 2.)
Teacher Resource
© 2021 College Board
98
ASSESS & REFLECT
Pre-AP Chemistry
UNIT 2
PRACTICE PERFORMANCE TASK
Properties of Limonene
OVERVIEW
DESCRIPTION
In this practice performance task, students reason
about intermolecular forces from data and a video
demonstration and predict relative strengths of
London dispersion forces based on molecular
structure. They then read about the extraction
of limonene and calculate yield. Finally, students
construct particulate models to model solutions
and mixtures and reason about partial pressure.
CONTENT FOCUS
This task is designed to assess students’ understanding
of molecules, solutions, and mixtures and to allow
them to practice their reasoning about intermolecular
forces and partial pressure from data. This task is
intended to be used after students have completed their
study of Key Concept 2.2: Molecular Structure and
Properties.
AREAS OF FOCUS
Attention to Modeling
Strategic Use of
Mathematics
Emphasis on Analytical
Reading and Writing
SUGGESTED TIMING
~45 minutes
HANDOUT
Unit 2 Practice
Performance Task:
Properties of Limonene
MATERIALS
calculator
equation sheet
periodic table
For Part 1, question 2:
LCD projector,
electronic whiteboard,
or other technology for
showing an online video
internet access to the
video demonstration
“Pop a Balloon with an
Orange Peel!” (0:29)
from the Chemical
Education Xchange at
https://www.chemedx.
org/blog/how-does-
orange-peel-pop-
balloon-chemistry-
course (first video on
the page)
Practice Performance Task: Properties of Limonene
CHEM_U2_TR_CONF.indd 98 29/01/20 6:46 PM

Course Guide
© 2021 College Board
Pre-AP Chemistry
49
Pre-AP Chemistry Assessments for Learning
About Pre-AP Chemistry
Student Resource
© 2021 College Board
Pre-AP Chemistry
91
Properties of Limonene
1. Limonene is a nonpolar substance found in citrus fruits such
as oranges and lemons. The structure of limonene is shown
at right.
Circle the term or terms below that apply to a sample of
limonene. (There may be more than one.) Justify your
answer.
Limonene
CH
2
CH
CH
3
CH
3
H
2
C
C
CH
C
H
2
C
H
2
C
atom compound element molecule mixture
2. Limonene has many interesting properties, one of which
involves its interaction with isoprene. Isoprene is the main
component of the rubber found in many kinds of balloons. The
structure of isoprene is shown at right.
Isoprene
CH
2
H
3
C
H
2
C
C C
H
You will watch a video demonstration that shows limonene
interacting with isoprene. Watch the demonstration and then
read and answer the following questions.
(a) After watching the demonstration, Angel, Kayla, and Jacob make the
following statements about what they observed:
Angel says, “I think limonene molecules repelled the isoprene molecules
since one kind of molecule is polar and the other is nonpolar. This
repulsion caused the balloon to pop.”
Kayla says, “I think the limonene particles hit the balloon with enough
force to pop it since the limonene particles are larger than the isoprene
particles.”
Jacob says, “I think the limonene particles dissolved the isoprene molecules
since they are both nonpolar particles and ‘like dissolves like.’”
Practice Performance Task: Properties of Limonene
PRACTICE
PERFORMANCE
TASK
Unit 2: Chemical Bonding and Interactions
CHEM_U2_SR_CONF.indd 91 29/01/20 6:43 PM

50
Course Guide
© 2021 College Board
Pre-AP Chemistry
Pre-AP Chemistry Assessments for Learning
About Pre-AP Chemistry
Student Resource
© 2021 College Board
Pre-AP Chemistry
92
Which student do you agree with and why? As part of your explanation,
comment on why the rationales of the other students are not valid.
(b) Based on your observations and your answer above, what type(s) of
intermolecular forces exist between molecules of isoprene? Justify your
answer.
(c) Which substance, limonene or isoprene, would you predict has a higher
boiling point? Justify your answer in terms of the intermolecular forces in
each substance.
Practice Performance Task: Properties of Limonene
PRACTICE
PERFORMANCE
TASK
Unit 2: Chemical Bonding and Interactions
CHEM_U2_SR_CONF.indd 92 29/01/20 6:43 PM

51
Pre-AP Chemistry Assessments for Learning
About Pre-AP Chemistry
Student Resource
© 2021 College Board
Pre-AP Chemistry
93
Practice Performance Task: Properties of Limonene
3. There are many ways to obtain limonene, which are discussed in the passage
below. Read the passage and answer the questions that follow.
This passage is excerpted and adapted from A. J. Andrews,
“How to Extract Oil from the Skin of Oranges.” ©2018 by
SFGate.
HOW TO EXTRACT OIL FROM THE SKIN
OF ORANGES
1 Orange peels are a source of the limonene, or
orange oil, you nd in many products, including
cosmetics, foods, and cleaning products.
Limonene is found in large concentrations close
to the surface of the peel. It can be released by
rubbing or heating.
2 Commercial oil producers have various methods
for extracting (or removing) the limonene from
oranges so it can be used in manufacturing. You
can replicate a couple of methods in your kitchen
using everyday tools. Most commercial producers
extract limonene from orange peels le over from
juicing oranges, using a method known as cold
extraction. Some producers use techniques such
as distillation or solvent extraction, and may work
with other parts of the plant in addition to just the
peels. Distillation yields about 150 milligrams of
oil per 15 grams of peel.
At-Home Cold Extraction
3 Cold pressing is one of the oldest methods of oil
extraction and one you can do at home with a
garlic press. You won’t get as much oil pressing
the peels as you would by distillation, but you’ll
extract enough for food avoring.
MY NOTES
Unit 2: Chemical Bonding and Interactions
CHEM_U2_SR_CONF.indd 93 29/01/20 6:43 PM

52
Course Guide
© 2021 College Board
Pre-AP Chemistry
Pre-AP Chemistry Assessments for Learning
About Pre-AP Chemistry
Student Resource
© 2021 College Board
Pre-AP Chemistry
94
4 To extract oil using a garlic press, rst scrape the
white pith from the inside of the orange peel.
Cut the peels into 1-inch pieces and heat them in
warm water (110 to 120 degrees Fahrenheit, or
43 to 49 degrees Celsius) on the stove; “cold” in
this sense means not heating the peels enough to
damage the oil. Pack the peels into the press and
squeeze the oil into a food container.
At-Home Solvent Extraction
5 Using ethanol to separate limonene from the
orange peels doesn’t require as much force as
pressing but it takes more time. Cut the peels
into 1/4- to 1/2-inch pieces and place them in
a clean glass jar. Add enough ethanol to barely
cover the peels and store the container in a room-
temperature cupboard for about two weeks.
Shake the container at least once a day. When it
is ready, strain the ethanol into a shallow dish.
Allow the ethanol to evaporate and scrape the oil
into a food container.
Practice Performance Task: Properties of Limonene
Unit 2: Chemical Bonding and Interactions
CHEM_U2_SR_CONF.indd 94 29/01/20 6:43 PM
MY NOTES
(a) As stated in the passage, cold extraction involves first heating orange peels to
110 to 120 degrees Fahrenheit (43 to 49 degrees Celsius). Why is this heating
useful in terms of the intermolecular forces of limonene in an orange peel?
(b) A perfume recipe calls for 2.0 g of orange oil, or limonene. Calculate how
many oranges you would need to peel to obtain 2.0 g of limonene using
distillation, according to the article. Assume that the peel from a large
orange has a mass of 85 g.

53
Pre-AP Chemistry Assessments for Learning
About Pre-AP Chemistry
Student Resource
© 2021 College Board
Pre-AP Chemistry
95
(c) Limonene is soluble in ethanol but insoluble in water. The table below shows
the densities of liquid ethanol, limonene, and water.
Substance Density of Liquid
(g/mL)
Ethanol 0.79
Limonene 0.84
Water 1.00
In the boxes below, draw particulate representations of the following two
mixtures:
A mixture of ethanol and limonene
A mixture of water and limonene
Use the key provided to represent particles of different substances. Draw at
least 5 particles of each substance in each diagram.
Mixture of ethanol and limonene
Mixture of water and limonene
ethanol
limonene
water
Key
Practice Performance Task: Properties of Limonene
TASK
Unit 2: Chemical Bonding and Interactions
CHEM_U2_SR_CONF.indd 95 29/01/20 6:43 PM

54
Course Guide
© 2021 College Board
Pre-AP Chemistry
Pre-AP Chemistry Assessments for Learning
About Pre-AP Chemistry
Student Resource
© 2021 College Board
Pre-AP Chemistry
96
(d) As stated in the passage, ethanol has to evaporate for the orange oil to be
isolated. A chemist takes a sample of the air directly above the evaporating
ethanol. A particulate representation of the sample is shown below.
= oxygen
= nitrogen
= ethanol
= limonene
The partial pressure of the nitrogen gas in the sample is 0.70 atm.
(i)
Calculate the total pressure of the gas sample.
(ii) Calculate the partial pressure of the ethanol in the sample.
1A
Practice Performance Task: Properties of Limonene
TASK
Unit 2: Chemical Bonding and Interactions
CHEM_U2_SR_CONF.indd 96 29/01/20 6:43 PM

55
Pre-AP Chemistry Assessments for Learning
About Pre-AP Chemistry
Teacher Resource
© 2021 College Board
103
ASSESS & REFLECT
Pre-AP Chemistry
UNIT 2
SCORING GUIDELINES
There are 25 possible points for this performance task.
Question 1
Sample Solutions Points Possible
The words compound and molecule
should be circled. Compounds are formed
when two or more different elements
are bonded together. Since limonene is a
covalent compound, the fundamental unit
is the molecule.
3 points maximum
1 point for circling compound
1 point for circling molecule
1 point for correct justification
Targeted Feedback for Student Responses
Refer students back to the card sort activity in which particles were classified at various
levels.
TEACHER NOTES AND REFLECTIONS
Practice Performance Task: Properties of Limonene
CHEM_U2_TR_CONF.indd 103 29/01/20 6:46 PM

56
Course Guide
© 2021 College Board
Pre-AP Chemistry
Pre-AP Chemistry Assessments for Learning
About Pre-AP Chemistry
Teacher Resource
© 2021 College Board
104
ASSESS & REFLECT
Pre-AP Chemistry
UNIT 2
Question 2, part (a)
Sample Solutions Points Possible
I agree with Jacob. Disrupting the
intermolecular forces between particles
of isoprene in the balloon causes a tiny
hole to form that allows the gases inside
to escape.
Angel is incorrect because the structures
of limonene and isoprene are similar in
terms of the type of bonds (only C and
H) that both have. Therefore, one can’t be
polar and the other nonpolar.
Kayla’s explanation does not make
sense, as balloons are often struck by a
variety of particles in motion, including
gas particles, without popping. Even
raindrops and grains of sand can hit a
balloon without popping it. So it’s not
clear why the limonene particles would
apply a stronger force than any of these
other things that are of a similar or larger
size.
4 points maximum
1 point for agreeing with Jacob
1 point for explaining why Jacob’s
rationale is the most logical in terms of
intermolecular forces
1 point for explaining why Angel’s
rationale is incorrect
1 point for explaining why Kayla’s
rationale is incorrect
Targeted Feedback for Student Responses
Remind students to think about what holds solid substances such as rubber together on
a molecular level.
TEACHER NOTES AND REFLECTIONS
Practice Performance Task: Properties of Limonene
CHEM_U2_TR_CONF.indd 104 29/01/20 6:46 PM

57
Pre-AP Chemistry Assessments for Learning
About Pre-AP Chemistry
Teacher Resource
© 2021 College Board
105
ASSESS & REFLECT
Pre-AP Chemistry
UNIT 2
Question 2, part (b)
Sample Solutions Points Possible
The forces between isoprene molecules
are London dispersion forces. Since
isoprene is nonpolar, it only experiences
London dispersion forces.
2 points maximum
1 point for identification of London
dispersion forces
1 point for correct justification
Targeted Feedback for Student Responses
Ask students to refer back to the lessons on intermolecular forces and ask them to
identify the type of intermolecular forces present in isoprene based on its structure.
TEACHER NOTES AND REFLECTIONS
Practice Performance Task: Properties of Limonene
CHEM_U2_TR_CONF.indd 105 29/01/20 6:46 PM

58
Course Guide
© 2021 College Board
Pre-AP Chemistry
Pre-AP Chemistry Assessments for Learning
About Pre-AP Chemistry
Teacher Resource
© 2021 College Board
106
ASSESS & REFLECT
Pre-AP Chemistry
UNIT 2
Question 2, part (c)
Sample Solutions Points Possible
I would predict that limonene has a
higher boiling point than isoprene.
Since both limonene and isoprene are
nonpolar, they both only have London
dispersion forces. The strength of
London dispersion forces depends on
the number of electrons. Limonene has
significantly more carbon and hydrogen
atoms than isoprene and, consequently,
has many more electrons that are
involved in London dispersion forces.
Thus, the intermolecular forces between
limonene molecules are greater, and the
energy needed to separate one limonene
molecule from another, as measured by
boiling point, would be greater.
3 points maximum
1 point for selection of limonene
1 point for explaining that stronger
London dispersion forces in limonene are
due to a greater number of electrons
1 point for discussing the relationship
of boiling point to the strength of
intermolecular forces
Targeted Feedback for Student Responses
Remind students to consider the factors that would increase the strength of
intermolecular forces for various types of molecules. They can refer back to the
evaporation lab and examine the trends they saw there.
TEACHER NOTES AND REFLECTIONS
Practice Performance Task: Properties of Limonene
CHEM_U2_TR_CONF.indd 106 29/01/20 6:46 PM

59
Pre-AP Chemistry Assessments for Learning
About Pre-AP Chemistry
Teacher Resource
© 2021 College Board
107
ASSESS & REFLECT
Pre-AP Chemistry
UNIT 2
Question 3, part (a)
Sample Solutions Points Possible
The increase in temperature would
cause the molecules to move around
more, overcoming intermolecular forces
between limonene and other substances
in the peel.
1 point maximum
1 point for a discussion of the agitation
of the molecules disrupting the
intermolecular forces between limonene
and other substances in the peel
Targeted Feedback for Student Responses
Ask students to recall what temperature actually measures and to consider where the
added energy goes when heating a substance.
TEACHER NOTES AND REFLECTIONS
Practice Performance Task: Properties of Limonene
CHEM_U2_TR_CONF.indd 107 29/01/20 6:46 PM

60
Course Guide
© 2021 College Board
Pre-AP Chemistry
Pre-AP Chemistry Assessments for Learning
About Pre-AP Chemistry
Teacher Resource
© 2021 College Board
108
ASSESS & REFLECT
Pre-AP Chemistry
UNIT 2
Question 3, part (b)
Sample Solutions Points Possible
1,000 mg limonene
2.0glimonene ××
1glimonene
15 gpeel 1orange
=2.4 oranges
150 mg limonene 85 gpeel
××
×
The recipe requires about 2.4 (or 3)
oranges.
3 points maximum
1 point for identifying the ratio of the
mass of peel to the mass of limonene
from the text
1 point for calculating the mass of
orange peel needed to extract 2.0 g
of limonene (could be implicit in the
calculation)
1 point for calculating the number of
oranges this would require
Scoring note: Students may give the
number of oranges as an integer, but
doing so is not required for credit.
Targeted Feedback for Student Responses
Students do not need to set up a dimensional analysis calculation, but they should use
proportional reasoning to arrive at a solution.
×
15 gpeel
150 mg limonene
1orange
85 gpeel
=2.4 oranges
2.0glimonene
1,000 mg limonene
1glimonene
TEACHER NOTES AND REFLECTIONS
Practice Performance Task: Properties of Limonene
CHEM_U2_TR_CONF.indd 108 29/01/20 6:46 PM

61
Pre-AP Chemistry Assessments for Learning
About Pre-AP Chemistry
Teacher Resource
© 2021 College Board
109
ASSESS & REFLECT
Pre-AP Chemistry
UNIT 2
Question 3, part (c)
Sample Solutions Points Possible
5 points maximum
Mixture of ethanol and limonene
1 point for showing a particulate
representation of at least 5 ethanol and 5
limonene molecules in a liquid state
1 point for a particulate-level
representation of the substances
completely dissolved in one another (no
layering of individual molecules)
Mixture of water and limonene
1 point for showing a particulate
representation of at least 5 water
molecules and 5 limonene molecules in
a liquid state
1 point for showing a particulate
representation of the lack of mixing of
the two different types of molecules
1 point for representing the water
molecules as the bottom layer and
limonene molecules as the top layer
Scoring note: Students do not need to
draw the liquid line.
Targeted Feedback for Student Responses
Help students think about how the density of the pure substances would manifest in a
mixture.
TEACHER NOTES AND REFLECTIONS
Practice Performance Task: Properties of Limonene
CHEM_U2_TR_CONF.indd 109 29/01/20 6:46 PM

62
Course Guide
© 2021 College Board
Pre-AP Chemistry
About Pre-AP Chemistry
Teacher Resource
© 2021 College Board
110
ASSESS & REFLECT
Pre-AP Chemistry
UNIT 2
Question 3, part (d)
Sample Solutions Points Possible
(i) There are a total of 15 gas particles in the
sample, 7 of which are nitrogen. Therefore,
7
15
of the total pressure of the sample is
due to the partial pressure of nitrogen.
P=
Χ
×
N
2
N
2
total
P
N
2
P =
total
Χ
N
2
0.70 atm
P =
total
7
15
P
=
1.5atm
total
P
(ii) Since ethanol particles make up
4
15
of the total number of gas particles in
the sample, they make up of the total
pressure.
4
15
P=
Χ
×
ethanolethanol total
P =
1.5atm
ethanol
×
15
4
P =
0.4atm
ethanol
P
4 points maximum
(i)
1 point for the relationship of the
number of nitrogen molecules to the
total number of molecules in the sample
1 point for correctly setting up and
solving the proportion for finding the
total pressure (students do not have to
explicitly use Dalton’s law)
(ii)
1 point for the relationship of ethanol
molecules to nitrogen molecules or
ethanol molecules to total number of
molecules in the sample
1 point for correctly setting up and
solving the proportion for finding the
pressure of ethanol (students do not
have to explicitly use Dalton’s law)
Scoring note: If a student calculates the
total pressure incorrectly in part (i) but
uses that pressure correctly in part (ii),
they can earn full credit for part (ii).
Targeted Feedback for Student Responses
Encourage students to show all their work and double-check that their answer makes
sense. If their answer doesn’t make sense, they could have made an algebra mistake.
TEACHER NOTES AND REFLECTIONS
Practice Performance Task: Properties of Limonene
CHEM_U2_TR_CONF.indd 110 29/01/20 6:46 PM
Pre-AP Chemistry Assessments for Learning

63
Pre-AP Chemistry Assessments for Learning
About Pre-AP Chemistry
Starting in the school year of 2021–22, Pre-AP Chemistry will include a final exam
featuring multiple-choice and technology-enhanced questions as well as an open-
response question. The final exam will be a summative assessment designed to measure
students’ success in learning and applying the knowledge and skills articulated in the
Pre-AP Chemistry Course Framework. The final exam's development follows best
practices such as multiple levels of review by educators and experts in the field for
content accuracy, fairness, and sensitivity. The questions on the final exam will be
pretested, and the resulting data will be collected and analyzed to ensure that the final
exam is fair and represents an appropriate range of the knowledge and skills of the
course.
The final exam will be delivered on a secure digital platform in a classroom setting.
Educators will have the option of administering the final exams in a single extended
session or two shorter consecutive sessions to accommodate a range of final exam
schedules.
Multiple-choice and technology-enhanced questions will be delivered digitally and
scored automatically with detailed score reports available to educators. This portion of
the final exam will build on the question styles and formats of the learning checkpoints;
thus, in addition to their formative purpose, the learning checkpoints provide practice
and familiarity with the final exam. The open-response question, modeled after
the performance tasks, will be delivered as part of the digital final exam but scored
separately by educators using scoring guidelines that are designed and vetted with the
question.
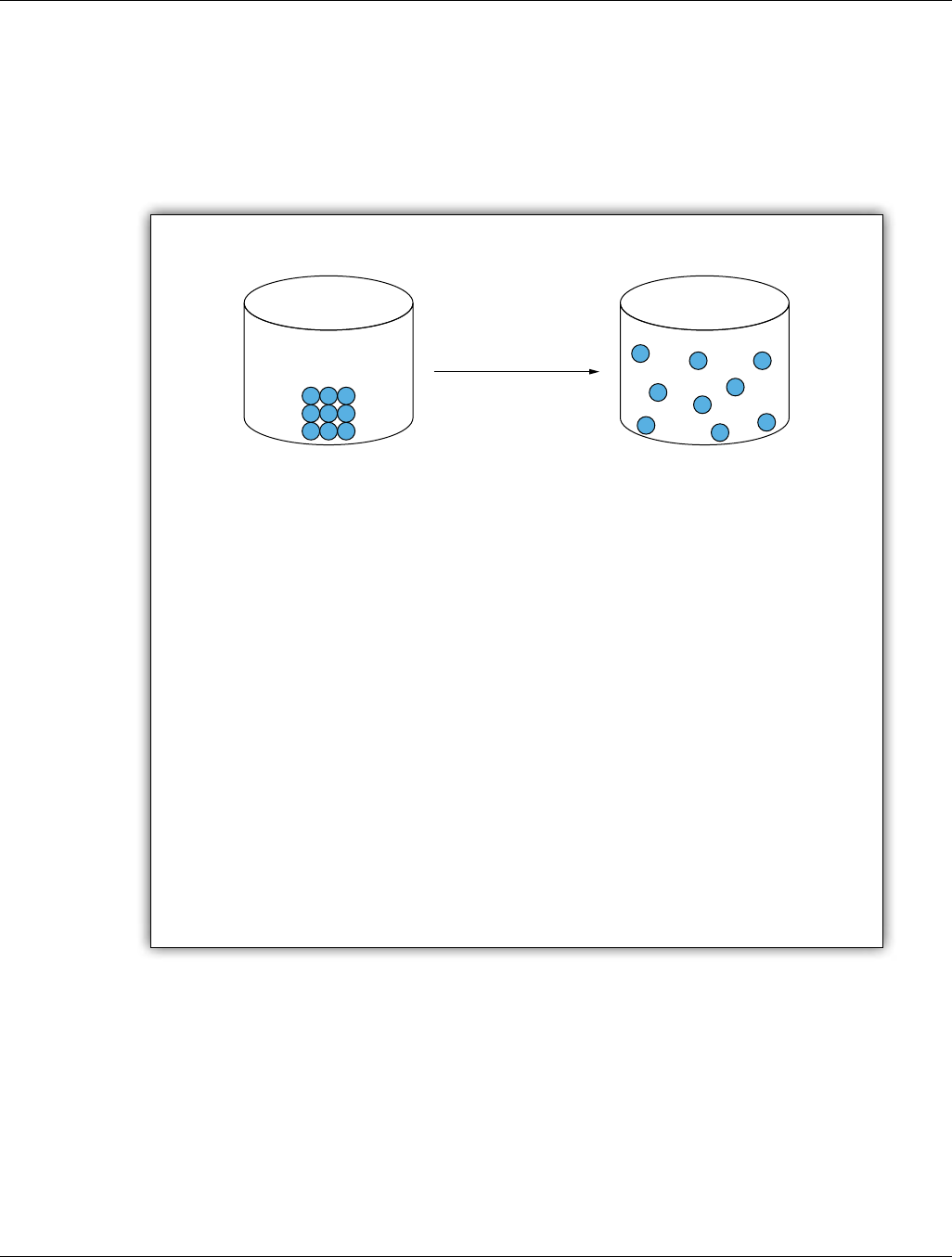
64
Course Guide
© 2021 College Board
Pre-AP Chemistry
Pre-AP Chemistry Assessments for Learning
About Pre-AP Chemistry
The following questions are representative of what students and educators will
encounter on the learning checkpoints and final exam.
1.
A student places 2.0 g of an unknown substance in a sealed container with no
other contents. The student observes the container for 10 minutes and then
draws the two particle diagrams shown to represent his observations of the
initial and final states.
Which of the following descriptions of the observed change is most consistent
with the model?
(A) e unknown substance underwent a phase change from solid to liquid and
the mass at the end of the experiment was 2.0 g.
(B) e unknown substance underwent a phase change from solid to liquid and
the mass at the end of the experiment was 1.5 g.
(C) e unknown substance underwent a phase change from solid to gas and
the mass at the end of the experiment was 2.0 g.
(D) e unknown substance underwent a phase change from solid to gas and
the mass at the end of the experiment was 1.5 g.

65
Pre-AP Chemistry Assessments for Learning
About Pre-AP Chemistry
Assessment Focus
Question 1 requires students to interpret a model of a phase change from solid to gas
and to reason based on their understanding of the conservation of mass.
Correct Answer: C
Learning Objective:
1.1.A.2 Describe how the properties of solids, liquids, and gases are related to particle
arrangement.
Area of Focus: Attention to Modeling
2.
Container 1 Container 2 Container 3
Xe Ne Xe + Ne
The figure represents three containers of equal volume at the same
temperature, each containing a different gas or mixture of gases.
Which TWO statements best describe the pressure in the containers?
(A) e pressure in Container 1 is greater than the pressure in Container 2
because the Xe atoms are larger than Ne atoms.
(B) e pressure in Container 1 is equal to the pressure in Container 2 because
they have the same number of atoms.
(C) e pressure in Container 3 is twice the pressure in Container 2 because
Container 3 has twice the number of atoms.
(D) e pressure in all three containers is the same because the containers are at
the same temperature.
(E) e pressure in all three containers is the same because the containers have
the same volume.

66
Course Guide
© 2021 College Board
Pre-AP Chemistry
Pre-AP Chemistry Assessments for Learning
About Pre-AP Chemistry
Assessment Focus
In question 2, students use a model to describe the relationship between the quantity
of a gas, or mixture of gases, and the resulting pressure. e question assesses
students’ understanding of partial pressure and the eect of temperature and volume
on gas pressure. is question also demonstrates the multiple-select question type that
students will encounter in learning checkpoints and the nal exam.
Correct Answers: B and C
Learning Objective:
2.1.C.1 Relate the total and partial pressure of a gas mixture to the number of
particles and their proportions.
Area of Focus: Attention to Modeling
3.
→4Na( )+O()2Na O( )
22
sg s
Sodium oxide is produced by the reaction of sodium metal with oxygen gas. The
reaction is represented by the chemical equation above. A chemical reaction is set
up with 32 moles of Na and 12 moles of O
2
in a reaction vessel. As the reaction
proceeds to completion, the number of moles of Na and O
2
are monitored and
plotted on a graph as shown in the figure.
Moles of reactants
8
12
16
20
24
28
32
4
Reaction progress
0
Na
O
2

67
Pre-AP Chemistry Assessments for Learning
About Pre-AP Chemistry
Which of the following best explains the steeper slope of the line that
represents moles of Na compared to the line that represents moles of O
2
?
(A) e initial mass of Na is greater than the initial mass of O
2
.
(B) For every mole of O
2
consumed in the reaction, 4 moles are Na are needed.
(C) e mass of 4 Na atoms is greater than the mass of 1 O
2
molecule.
(D) e initial number of moles of Na is greater than the initial number of moles
of O
2
.
Assessment Focus
In question 3, students analyze data about the rate at which reactants in a chemical
reaction are used and explain the dierence based on the stoichiometry of the
balanced chemical equation. All four multiple-choice options are correct statements,
but only option B provides an explanation for the dierence in slope.
Correct Answer: B
Learning Objective:
3.2.B.1 Explain the relationship between the quantity of reactants consumed and the
quantity of products formed in a chemical transformation.
Area of Focus: Strategic Use of Mathematics

68
Course Guide
© 2021 College Board
Pre-AP Chemistry
Pre-AP Chemistry Assessments for Learning
About Pre-AP Chemistry
4.
Immediately after
acid is added
After acid-base
reaction is complete
H
2
O
unknown acid
The figure shows particle diagrams representing a 13M solution of an
unknown acid after the acid was added to water.
Based on the particle diagrams, which of the following claims about the
unknown acid is most likely correct?
(A) e acid is strong because there are 13 moles of acid in every liter of water.
(B) e acid is strong because all the acid molecules dissociate in solution.
(C) e acid is weak because it produces only one hydronium ion per acid
molecule in solution.
(D) e acid is weak because there are fewer acid molecules than water
molecules in the solution.
Assessment Focus
In question 4, students evaluate a model of a dissociated acid and use the model to
support a claim about the strength of the acid. e question is designed to address
the common challenge students have in distinguishing between strong acids and
concentrated acids.
Correct Answer: B
Learning Objectives:
4.3.A.1 Create and/or evaluate models of strong and weak acids and bases.
4.3.A.2 Distinguish between strong and weak acids in terms of degree of dissociation
in aqueous solution.
Area of Focus: Attention to Modeling

69
About Pre-AP Chemistry
Pre-AP Chemistry Course Designation
Schools can earn an official Pre-AP Chemistry course designation by meeting the
requirements summarized below. Pre-AP Course Audit Administrators and teachers
will complete a Pre-AP Course Audit process to attest to these requirements. All schools
offering courses that have received a Pre-AP Course Designation will be listed in the
Pre-AP Course Ledger, in a process similar to that used for listing authorized AP courses.
The school ensures that Pre-AP frameworks and assessments serve as the
foundation for all sections of the course at the school. This means that the school
must not establish any barriers (e.g., test scores, grades in prior coursework,
teacher or counselor recommendation) to student access and participation in the
Pre-AP Chemistry coursework.
Teachers have read the most recent Pre-AP Chemistry Course Guide.
Teachers administer each performance task and at least one of two learning
checkpoints per unit.
Teachers and at least one administrator per site complete a Pre-AP Summer
Institute or the Online Foundational Module Series. Teachers complete at least one
Online Performance Task Scoring Module.
Teachers align instruction to the Pre-AP Chemistry Course Framework and ensure
their course meets the curricular requirements summarized below.
The school ensures that the resource requirements summarized below are met.
The course provides opportunities for students to develop understanding of the
Pre-AP Chemistry key concepts and skills articulated in the course framework
through the four units of study.
The course provides opportunities for students to engage in the Pre-AP shared
instructional principles.
u
close observation and analysis
u
evidence-based writing
u
higher-order questioning
u
academic conversation

70
Course Guide
© 2021 College Board
Pre-AP Chemistry
Pre-AP Chemistry Course Designation
About Pre-AP Chemistry
The course provides opportunities for students to engage in the three Pre-AP
science areas of focus. The areas of focus are:
u
emphasis on analytical reading and writing
u
strategic use of mathematics
u
attention to modeling
The instructional plan for the course includes opportunities for students to
continue to practice and develop disciplinary skills.
The instructional plan reflects time and instructional methods for engaging
students in reflection and feedback based on their progress.
The instructional plan reflects making responsive adjustments to instruction based
on student performance.
The school ensures that participating teachers and students are provided computer
and internet access for completion of course and assessment requirements.
Teachers should have consistent access to a video projector for sharing web-based
instructional content and short web videos.
The school ensures teachers have access to laboratory equipment and consumable
resources so that students can engage in the Pre-AP Chemistry inquiry-based
model lessons.

71
About Pre-AP Chemistry
Accessing the Digital Materials
Pre-AP Classroom is the online application through which teachers and students can
access Pre-AP instructional resources and assessments. The digital platform is similar
to AP Classroom, the online system used for AP courses.
Pre-AP coordinators receive access to Pre-AP Classroom via an access code delivered
after orders are processed. Teachers receive access after the Pre-AP Course Audit
process has been completed.
Once teachers have created course sections, student can enroll in them via access
code. When both teachers and students have access, teachers can share instructional
resources with students, assign and score assessments, and complete online learning
modules; students can view resources shared by the teacher, take assessments, and
receive feedback reports to understand progress and growth.

Appendix


75
Pre-AP Chemistry Equations, Constants, and Tables of Information
Appendix
Units
Symbol
L liter(s)
g gram(s)
atm atmosp
here(s)
Pa pascal(s)
mm Hg millimeters of mercury
J joule(s)
mol mole(s)
K kelvin
M
molarity
cal calorie(s)
Polyatomic Ions
Formula
acetate
−
CH COO
3
or
CHO
232
–
ammonium
NH
4
+
bicarbonate or
hydrogen carbonate
HCO
–
3
carbonate
CO
3
2–
chromate
CrO
4
2–
cyanide
CN
–
dichromate
Cr O
27
2–
hydroxide
OH
–
nitrate
NO
3
–
nitrite
NO
2
–
phosphate
PO
4
3–
sulfate
SO
4
2–
sulte
SO
3
2–
Constants
Constant Value
Avogadro’s number
×6.02 10
23
particles per mole
Gas constant, R
0.0821
L•atm
mol•K
Specic heat capacity
of
HO()
2
l
4.18
J
g•K
Standard temperature
and pressure
273 K and 1 atm
Metric Prefixes
Factor Symbol
10
3
kilo k
−
10
2
centi c
−
10
3
milli m
−
10
6
micro μ
−
10
9
nano n
Conversions
1 atm = 760 mm Hg = 760 torr = 101 kP
a
1 cal = 4.18 joules
0°C = 273 K
Activity Series
most
easily
oxidized
least
easily
oxidized
Metals
Li
K
Ba
Ca
Na
Mg
Al
Mn
Zn
Cr
Fe
Co
Ni
Sn
Pb
(H
2
)
Cu
Hg
Ag
Pt
Au
Solubility Guidelines
All sodium, potassium,
ammonium, and nitrate salts
are soluble in water.

76
Course Guide
© 2021 College Board
Pre-AP Chemistry
Equations
Density
=
m
V
D
D = density
m = mass
V = volume
Percent error
=×percenterror
|accepted value–experimental value|
accepted value
100
Percent yield
=×percentyield
actual yield
theoreticalyield
100
Molarity
=molarity
molesofsolute
literofsolution
Gas laws
PV
T
PV
T
=
11
1
22
2
PX P×
=
AA total
PPPP
=+
++
totalABC
…
PnRTV =
P = pressure
V = volume
T = temperature
n = moles of gas
R = gas constant
X = fraction of the gas
Heat
∆=qmcT
q = heat
m = mass
c = specic heat capacity
∆T
= change in temperature
pH
+
pH =–log[HO ]
3
Appendix

77
1 18
1
H
Hydrogen
1.008
2 13 14 15 16 17
2
He
Helium
4.00
3
Li
Lithium
6.94
4
Be
Beryllium
9.01
5
B
Boron
10.81
6
C
Carbon
12.01
7
N
Nitrogen
14.01
8
O
Oxygen
16.00
9
F
Fluorine
19.00
10
Ne
Neon
20.18
11
Na
Sodium
22.99
12
Mg
Magnesium
24.30
3 4 5 6 7 8 9 10 11 12
13
Al
Aluminum
26.98
14
Si
Silicon
28.09
15
P
Phosphorus
30.97
16
S
Sulfur
32.06
17
Cl
Chlorine
35.45
18
Ar
Argon
39.95
19
K
Potassium
39.10
20
Ca
Calcium
40.08
21
Sc
Scandium
44.96
22
Ti
Titanium
47.87
23
V
Vanadium
50.94
24
Cr
Chromium
52.00
25
Mn
Manganese
54.94
26
Fe
Iron
55.85
27
Co
Cobalt
58.93
28
Ni
Nickel
58.69
29
Cu
Copper
63.55
30
Zn
Zinc
65.38
31
Ga
Gallium
69.72
32
Ge
Germanium
72.63
33
As
Arsenic
74.92
34
Se
Selenium
78.97
35
Br
Bromine
79.90
36
Kr
Krypton
83.80
37
Rb
Rubidium
85.47
38
Sr
Strontium
87.62
39
Y
Yttrium
88.91
40
Zr
Zirconium
91.22
41
Nb
Niobium
92.91
42
Mo
Molybdenum
95.95
43
Tc
Technetium
44
Ru
Ruthenium
101.07
45
Rh
Rhodium
102.91
46
Pd
Palladium
106.42
47
Ag
Silver
107.87
48
Cd
Cadmium
112.41
49
In
Indium
114.82
50
Sn
Tin
118.71
51
Sb
Antimony
121.76
52
Te
Tellurium
127.60
53
I
Iodine
126.90
54
Xe
Xenon
131.29
55
Cs
Cesium
132.91
56
Ba
Barium
137.33
57–71
*
72
Hf
Hafnium
178.49
73
Ta
Tantalum
180.95
74
W
Tungsten
183.84
75
Re
Rhenium
186.21
76
Os
Osmium
190.23
77
Ir
Iridium
192.22
78
Pt
Platinum
195.08
79
Au
Gold
196.97
80
Hg
Mercury
200.59
81
Tl
allium
204.38
82
Pb
Lead
207.2
83
Bi
Bismuth
208.98
84
Po
Polonium
85
At
Astatine
86
Rn
Radon
87
Fr
Francium
88
Ra
Radium
89–103
†
104
Rf
Rutherfordium
105
Db
Dubnium
106
Sg
Seaborgium
107
Bh
Bohrium
108
Hs
Hassium
109
Mt
Meitnerium
110
Ds
Darmstadtium
111
Rg
Roentgenium
112
Cn
Copernicium
113
Nh
Nihonium
114
Fl
Flerovium
115
Mc
Moscovium
116
Lv
Livermorium
117
Ts
Tennessine
118
Og
Oganesson
57
La
Lanthanum
138.91
58
Ce
Cerium
140.12
59
Pr
Praseodymium
140.91
60
Nd
Neodymium
144.24
61
Pm
Promethium
62
Sm
Samarium
150.36
63
Eu
Europium
151.97
64
Gd
Gadolinium
157.25
65
Tb
Terbium
158.93
66
Dy
Dysprosium
162.50
67
Ho
Holmium
164.93
68
Er
Erbium
167.26
69
Tm
ulium
168.93
70
Yb
Ytterbium
173.05
71
Lu
Lutetium
174.97
89
Ac
Actinium
90
orium
232.04
91
Pa
Protactinium
231.04
92
U
Uranium
238.03
93
Np
Neptunium
94
Pu
Plutonium
95
Am
Americium
96
Cm
Curium
97
Bk
Berkelium
98
Cf
Californium
99
Es
Einsteinium
100
Fm
Fermium
101
Md
Mendelevium
102
No
Nobelium
103
Lr
Lawrencium
*Lanthanoids
†
Actinoids
Appendix

INCLUDES
Approach to
teaching and
learning
Course map
Course framework
Sample
assessment
questions
Pre-AP
®
Chemistry
COURSE GUIDE
preap.org/Chemistry-CG
© 2021 College Board. 01560-064
01560-064-Pre-AP-Covers-3P.indd 79-80 3/27/20 1:38 PM


