
Statistical Process Analysis using
NWA Quality Analyst software
and Agilent OpenLAB
ECM Intelligent Reporter
Application Note
Abstract
Statistical Process Control (SPC) is a well-established element of ISO 9001 certified
quality management systems. Northwest Analytics (NWA) Quality Analyst software offers
advanced statistical functions to analyze chromatography data system (CDS) result data
stored in Agilent OpenLAB ECM. This note describes how NWA Quality Analyst software
could be used with OpenLAB ECM and ECM Intelligent Reporter.
Introduction
Quality assurance and quality improvement
are important elements of ISO 9000
Quality management systems
1
. Statistical
Process Control (SPC), Process capability
analysis, process performance analysis, and
regression analysis are important statistical
techniques helping you not only to achieve
planned results but also to continually
improve your production and lab processes
2
.
While processes operate within
specifications they still might be out of
statistical control resulting in random out-of-
spec situations.
SPC helps identify single event or systemic
reasons for deviations and uncover
opportunities for process improvements
3
.
This is not only limited to manufacturing,
chemical, or pharmaceutical production
processes, but it can be applied to general
lab operation and method validation
processes. According to the ISO / IEC
17025: “data shall be recorded in such
a way that trends can be detected and
statistical techniques can be applied
to review Results”. SPC can also help
laboratory‘s top management to “conduct
a review of the … testing and/or calibration
activities to ensure their continuing suitability
and effectiveness”
4,5
.
To get the best understanding of laboratory
trends, large amounts of data, collected over
months or years will provide a higher level
of statistical significance. With the Agilent
OpenLAB ECM Intelligent Reporter result
database analytical quantitation results
generated by Agilent OpenLAB CDS or
Waters Empower can be stored for several
years - across multiple labs and across larger
time ranges.
The Agilent Report Template Editor or SQL
Server Report Builder allows you to generate
e.g. method, instrument and/or compound
specific result charts (see figure1). Microsoft
SQL server Reporting Services technology
refreshes and publishes web-based reports
in an unattended mode.
When developing new processes, or
improving or troubleshooting existing
processes you need to handle your data
in a more interactive way and apply
various statistical methods to find the
most applicable one. Amongst a variety of
software packages for advanced statistics,
this note describes how to use NWA
Quality Analyst with Agilent OpenLAB ECM
Intelligent Reporter. The setup is described
in an associated technical note Connecting
NWA Quality Analyst software to Agilent
OpenLAB ECM Intelligent Reporter
6
Software requirements
Visit http://www.nwasoft.com/products/
nwa-quality-analyst to request a free trail
copy of NWA Quality Analyst.
Please write to Dana Petrusich (dpetrusich@
nwasoft.com) for additional sales
information.
Data backend:
Agilent OpenLAB ECM 3.4.1 with
Agilent OpenLAB ECM Intelligent Reporter
A.02.0x
Supported CDS systems:
Agilent OpenLAB CDS rev. A.01.03 or higher
Waters
®
Empower
®
2 or 3

2
Functionality of NWA Quality
Analyst in sum
Complete SPC Charting – Includes
variable and attribute control charts, process
capability analysis and process based
analytics and visualization.
Charting Automation – Procedures easily
automated with wizard based “Run File”
scripting. Routine charting operations can
be called from OpenLAB ECM client with a
single icon.
Examples
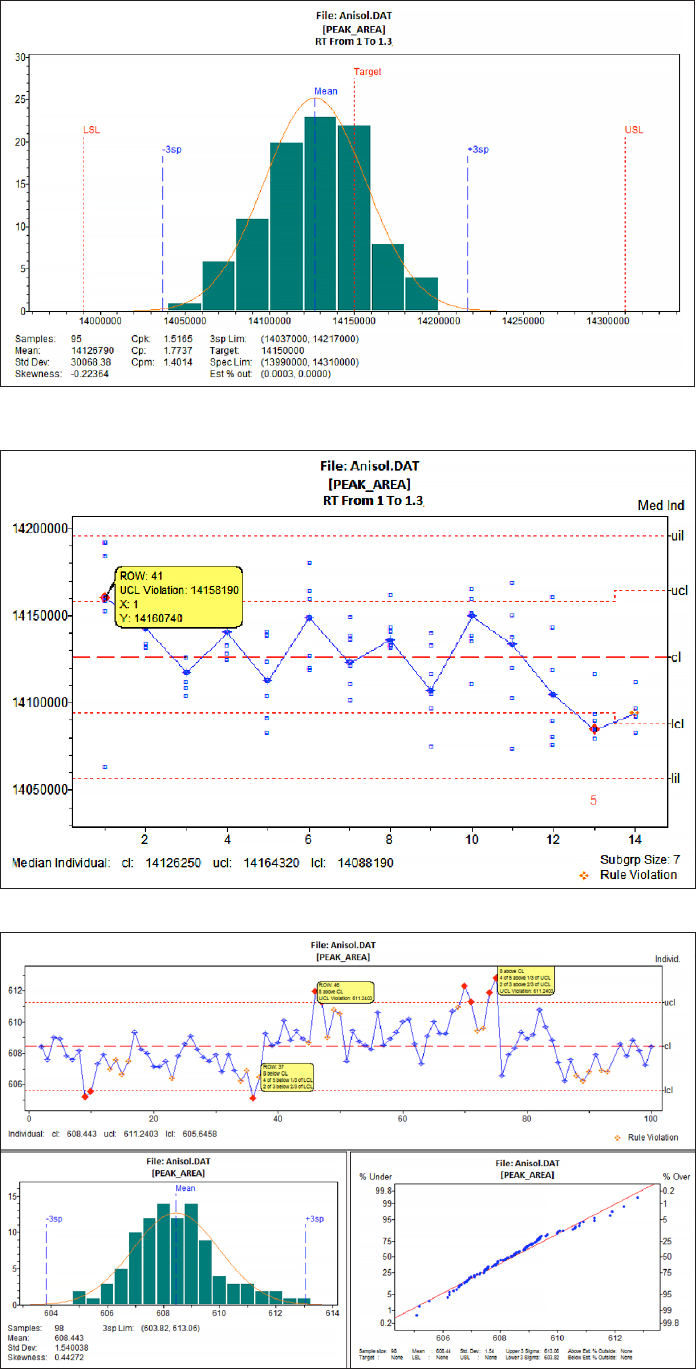
Figure 2 shows the result of a process
capability analysis. The detected peak area
for Anisol in a series of 95 analyses is plotted
in a histogram to visualize the distribution
type of the result data. A normal distribution
is a requirement for applying basic tools of
SPC. The diagram shows also the target,
and the Upper and Lower Specification limits
(USL, LSL) of the process. The capability
index C
p
is calculated by comparing the
width of the specification range with the
range of a short term 6σ band. If the ratio is
greater 1.5 the process is considered to be
“capable” and the potential for the analysis
to meet specifications good.
The C
pk
value relates the closer specification
limit to the mean value. It takes into account
that processes are not always perfectly
centered and bigger differences in C
p
and
C
pk
indicate an opportunity for process
improvement
3
.
Figure 3 shows a control chart based
on subgroups of 7 analyses. Individual
values and medians of each subgroup
are plotted against the number of groups.
Since standard deviations become smaller
with increasing numbers of samples, the
Upper and Lower Control limits (UCL, LCL)
are calculated based on a fixed number
of samples. They are adjusted if the
subgroup contains less than 7 data points to
compensate for the expected difference.
Deviations potentially related to a single
event (“special cause”) are indicated by red
dots and tool tips provide information about
the violated evaluation rule. Selection of
evaluation rules and control limits requires
good knowledge of the underlying statistical
rules in order to prevent false alarms
3
.
Figure 4 combines an individual value
control chart with a histogram and a normal
probability plot (Q-Q plot). The normal
probability plot visualizes the deviations from
a theoretical normal distribution of results.
Although a histogram of normal distribution
may be within specification limits, it does
not show the history of data collection and
possible trends.
Figure 1. ECM Intelligent Reporter: Individual values control chart with trend and imbalance
notification (Shewart rules)
Exception Reports – Reports can be
configured to show exceptions based
on specification, SPC and pattern rule
violations. Includes integrated Assignable
Cause / Corrective Action logging.
Industry and Application Specific
SPC Modules – Specialized modules such
as Multivariate SPC and Stability Analytics
add additional capabilities to
NWA Quality Analyst.
3
Figure 2. NWA Quality Analyst:
Histogram plot of Anisol peak areas
showing the distribution pattern of
95 analysis results. The width of
the specification range (USL-LSL) in
comparison to the calculated 6sp range
is a measure for process capability,
expressed as capability indices C
p
.
Figure 3. NWA Quality Analyst:
Control chart based on the average of
7 individual values for each subgroup.
An upper control limit rule violation is
indicated by a “Rule Violation” marker.
A tooltip shows details of the violation.
Implementation of Shewart or Westguard
rules are possible, too
Figure 4. NWA Quality Analyst:
Individual values control chart,
histogram plot, and normal probability
plot can be combined in a single layout.

4
Integration of NWA Quality
Analyst to OpenLAB ECM
clients
Generation of pre-defined control charts can
be triggered directly from the OpenLAB ECM
client. Further automation using OpenLAB
ECM Business Process Manager (BPM) is
possible. Quality Analyst software operates
with various different types of files:
*.DAT files contain the data used to
generate the charts. The *.DAT files could be
stored permanently or just temporarily.
*.NWH files: In case of ECM Intelligent
reporter, the database connection and query
can be stored in this file type. The NWH file
gets generated when saving a new .DAT file.
*.RUN files are script files that could be
used to launch a database connection, call a
.DAT file (and the corresponding .NWH file)
and generate a chart designed by the Run
File Wizard of Quality Analyst
*.NWG files contain an interactive snapshot
of a chart. Storing *.NWH and *.RUN files
in ECM is sufficient to preserve a specific
database query and chart layout. *.DAT files
can always be generated by double-clicking
*.NWH files.
Automated chart generation:
By double-clicking a specific .RUN file
the Quality Analyst software connects to
the database, retrieves the latest data
(dependent on the query definition) and
generates a single or multiple charts as
defined by the run file. This interactive chart
can be stored as an interactive .NWG file or
in any other graphics format (*.png, *.jpg,
*.wmf,*.bmp, *.gif, *.pcx). The chart can be
uploaded to ECM to store the history of all
control charts.
Interactive chart generation:
By double-clicking a specific .NWH file
the Quality Analyst software connects to
the database, retrieves the latest data
(dependent on the query definition) and
shows the data in the Quality Analyst
application window.
The application window allows further
filtering of the data, modification of the
database query and generating all kind of
sophisticated statistics and charts.
Figure 5. Quality Analyst run files stored in OpenLAB ECM
Figure 6. NWA Quality Analyst with query results from OpenLAB ECM Intelligent Reporter

References
1. International Organization for Standardization ISO 9000:2005: Quality management
systems - Fundamentals and vocabulary,
2. International Organization for Standardization ISO/TR 10017:2003: Guidance on
statistical techniques for ISO 9001:2000
3. Joglekar, Anand M.: Statistical methods for six sigma, 2003, Wiley (ISBN
0-471-20342-4)
4. L. Huber: Understanding and Implementing ISO/IEC 17025, 2009, Agilent Technologies
(P/N 5990-4540EN)
5. International Organization for Standardization ISO/IEC 17025:2005: General requirements
for the competence of testing and calibration laboratories
6. Agilent Technologies: Connecting NWA Quality Analyst software to Agilent OpenLAB
ECM Intelligent Reporter (P/N 5991-5439EN)
7. Carson P., Dent N.: Good Clinical, Laboratory and Manufacturing Practices:
Techniques for the QA Professional, 2007, The Royal Society of Chemistry, (ISBN:
978-0-85404-834-2)
8. E. Prichard, V. Barwick: Quality Assurance in Analytical Chemistry, 2007, Wiley (ISBN:
978-0-470-51776-5)
9. Agilent Technologies: Building valuable reports with your Waters Empower Data,
Technical Overview (P/N 5991-1642EN)
10. Agilent Technologies: Supporting Waters Empower 2 Data with OpenLAB Enterprise
Content Manager, Data Sheet (P/N 5990-3240EN)
www.agilent.com/chem/openlab
For research use only. Not for use in diagnostic
procedures. This information is subject to change
without notice.
© Agilent Technologies, Inc., 2015
Published in USA, January 23, 2015
Publication Number 5991-5440EN
