
REVIEW
published: 16 February 2021
doi: 10.3389/fmed.2021.622225
Frontiers in Medicine | www.frontiersin.org 1 February 2021 | Volume 8 | Article 622225
Edited by:
Savino Sciascia,
University of Turin, Italy
Reviewed by:
Claudio Ponticelli,
Retired, Italy
Jose Inciarte-Mundo,
Hospital Clínic de Barcelona, Spain
*Correspondence:
Isabelle Ayoub
Specialty section:
This article was submitted to
Rheumatology,
a section of the journal
Frontiers in Medicine
Received: 28 October 2020
Accepted: 20 January 2021
Published: 16 February 2021
Citation:
Mejía-Vilet JM and Ayoub I (2021) The
Use of Glucocorticoids in Lupus
Nephritis: New Pathways for an Old
Drug. Front. Med. 8:622225.
doi: 10.3389/fmed.2021.622225
The Use of Glucocorticoids in Lupus
Nephritis: New Pathways for an Old
Drug
Juan M. Mejía-Vilet
1
and Isabelle Ayoub
2
*
1
Department of Nephrology and Mineral Metabolism, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán,
Mexico, Mexico,
2
Division of Nephrology, Department of Internal Medicine, The Ohio State University Wexner Medical Center,
Columbus, OH, United States
Glucocorticoids therapy has greatly improved the outcome of lupus nephritis patients.
Since their discovery, their adverse effects have counterbalanced their beneficial
anti-inflammatory effects. Glucocorticoids exert their effects through both genomic
and non-genomic pathways. Differential activation of these pathways is clinicall y
relevant in terms of benefit and adverse effects. Ongoing aims in lupus nephritis
treatment development focus on a better use of glucocorticoids combined with
immunosuppressant drugs and biologics. Newer regimens aim to decrease the peak
glucocorticoid dose, allow a rapid glucocorticoid tapering, and intend to control disease
activity with a lower cumulative glucocorticoid exposure. In this review we discuss the
mechanisms, adverse effects and recent strategies to limit glucocorticoid exposure
without compromising treatment efficacy.
Keywords: glucocorticoids, lupus nephritis, systemic lupus erythematosus, prednisone, methylpredisolone,
steroids, adverse effect
INTRODUCTION
Cortisone (“compound E” or 17-hydroxy-11-dehydrocorticosterone) was identified in the 1930’s
by Edward Kendall and Tadeusz Reichstein, and later purified and synthesized in the 1940’s.
Compound E had strong anti-inflammatory effects but also potent mineralocorticoid effects which
manifested as fluid retention, hypertension, and hypokalemia. Compound E was first applied
for treatment in 1948 by Philip Hench. At that time, a young woman with severe rheumatoid
arthritis would become the first pat ient treated with cortisone. The anti-inflammatory effects of
the cortisone were remarkable but so were the adverse effects (
1, 2).
Subsequently, other glucocorticoid (GC) preparations were developed for treating autoimmune
diseases, including systemic lupus eryt hematosus (SLE) (3). The use of these anti-inflammatory
steroids in lupus nephritis (LN) dramatically improved survival. For example, survival was 17%
at 5 years in the pre-glucocorticoid era, but 55% at 5 years after introduction of glucocorticoids
(
4, 5). The addition of immunosuppressive drugs to GC, and later on, the development of biologic
drugs, have transitioned LN management to one focused on improving kidney outcomes while
minimizing adverse events. In this review, we discuss the use of GCs from mechanisms, adverse
events to management of lupus nephritis, and current strategies to limit toxicity of these drugs.

Mejía-Vilet and Ayoub The Use of Glucocorticoids in Lupus Nephritis
MECHANISM OF ACTION: THE CLINICAL
RELEVANCE OF THE GENOMIC AND
NON-GENOMIC MECHANISMS
Glucocorticoids are involved in regulatory processes throughout
the body, such as energy and lipid metabolism, and adaptation
to stress. Two of their most important effects are their strong
anti-inflammatory and immunosuppressive effects, evident at
concentrations above the physiological glucocorticoid levels (6).
Glucocorticoids and synthetic glucocorticoids have two
mechanisms of action: the genomic and non-genomic
mechanisms (Figure 1) (7). Genomic mechanisms are activated
after GC, as lipophilic molecules, cross the cell membranes
and bind to the multiprotein complex of chaperones (e.g.,
Hsp40, Hsp56, Hsp70, and Hsp90), immunophilins that act as
co-chaperones (e.g., p23, FKBP51, FKBP52), and the intracellular
cytoplasmic glucocorticoid receptor (cGR). After binding and
subsequent dissociation from these proteins, the complex
GC-cGR translocates to the nucleus and binds to DNA binding
sites known as glucocorticoid response elements. The final result
is a decreased transcription of genes encoding inflammatory
cytokines (e.g., interleukin-6, i nterleukin-8, tumor necrosis
factor-a), a process known as transrepression; and an increased
transcription of anti-inflammatory genes (e.g., interleukin-10,
IκB, annexin A1), known as transactivation (
8).
Genomic me chanisms are generally evident 30 min after
GC administration. By contrast, a second type of non-
genomic mechanisms produce effects within minutes after the
administration. These non-genomic effects are mediated through
changes in cellular membranes, inactivation of the phospholipase
A2 enzyme, and interaction with membrane glucocorticoid
receptors (mGR). Second messengers include kinases, such as the
p38 MAP kinase. The final effect is decreased lymphocyte activity
and proliferation (
9).
Identification of genomic and non-genomic mechanisms
is clinically important due to the differential adverse effect
profile and differential activation exerted by currently used
glucocorticoid dosages and preparations (Figure 1). Genomic
effects are activated with low (<7.5 mg prednisone equivalent
per day) to moderate (7.5–30 mg prednisone equivalent per
day) GC doses, and cGRs a re progressively saturated wit h
high-doses above 30 to 50 mg per day (10). From this
pharmacologic concept, prednisone doses above 50 mg per
day approach the ceiling of cGR saturation, with limited
additional anti-inflammatory benefit, yet increasing th e risk
for adverse effect s. As will be further discussed, some adverse
effects, such as avascular bone necrosis, are dependent on the
peak GC dose and duration of high-dose exposure (tapering
speed) (Figure 2).
Non-genomic mechanisms are activated with very-high GC
dosages, such as those reached with methylprednisolone pulses.
This activation starts at prednisone dosages of 100 mg, and
reaching its maximum around 250 to 500 mg. In contrast
to effects mediated by genomic mechanisms, non-genomic
mechanisms are thought to be associated with less adverse effects,
at le ast in part due to the short duration of administration (
11).
The relative activation of these genomic and non-genomic
pathways differs among different GC preparations. For example,
dexamethasone and methylprednisolone activate the non-
genomic pathway at a 3-fold greater rate than prednisone (
12).
Different GC preparations also differ in potency (expressed
relative to hydrocortisone), mineralocorticoid effects, and
duration of suppression of the hypothalamic-pituitary-adrenal
axis (
13). Other factors, such as time of administration (less
suppression when administered in the morning) and their
chronopharmacology, contribute to the degree of GC-axis
suppression and in consequence to the severity of adverse effects,
but are beyond the scope of this review (14).
Understanding these mechanisms is important to de ve lop
strategies to limit GC toxicity. As shown in Figure 2, GC
administration strategies used in recent clinical trials have
included intravenous methylprednisolone pulses, which activate
non-genomic pathways, followed by lower peak oral GC dosages
and a faster tapering of oral GCs. This strategy aims to maintain
treatment efficacy while limiting GC-related adverse effects.
GLUCOCORTICOID-RELATED ADVERSE
EVENTS
Both disease activity and glucocorticoid exposure have been
associated with organ damage in SLE (
15, 16). As patients
with higher degree of disease activity are usually treated with
higher GC doses, many of the reported studies suffer of
confounding by indication (i.e., patients with more severe activity
are administered higher GC doses). Also, as damage is frequently
measured through indices that group several manifestations
[e.g., the SLIC C/ACR damage index (SDI)], it is difficult to
distinguish organ damage caused by prednisone from that
caused by disease activity or concomitant immunosuppressive
medications (17). Finally, many studies also suffer from time
bias, as the contribution of disease activity to da mage is usually
higher at earlier stages, while GC-related damage is greater at
later stages (
17).
Organ damage occurs in 50% of patients with SLE within 5-
years of SLE diagnosis (18), with reported increased risk of 2.8%
for each 1 mg prednisone per day (19). Organ da mage has been
reported to be minimized by achieving disease remission (15, 20),
and by using maintenance doses of prednisone lower than 6.0 to
7.5 mg per day (21–23).
GC-related adverse effects have also been classified into
those related to high dosing over a short period of time, and
those related to cumulative GC doses. Table 1 summarizes t he
reported GC adverse effects according to the use of intravenous
methylprednisolone pulses, the peak oral-GC dose, the duration
of exposure to high-GC doses, and the GC cumulative dose.
INFECTIONS
Infections have been frequently associated with the peak dose of
GC and the duration of exposure to high GC doses. Infections
continue to be a major cause of hospitalizat ion and mortality in
Frontiers in Medicine | www.frontiersin.org 2 February 2021 | Volume 8 | Article 622225

Mejía-Vilet and Ayoub The Use of Glucocorticoids in Lupus Nephritis
FIGURE 1 | Genomic and non-genomic mechanisms of glucocorticoids. Glucocorticoid genomic pathway is mediated through the cytoplasmic glucocorticoid
receptor (cGR) leading to the mechanisms of gene transactivation and transrepression. The non-genomic pathway is mediated through the membrane glucocorticoid
receptor (mGR), inhibition of the phospholipase A2, and changes in cell membranes. The arrow in the left depicts the dose of prednisone required to activate these
pathways. The upper and lower gray zones represent the doses were genomic (lower gray zone) and non-genomic (upper gray zone) are fully saturated without added
benefit and with higher incidence of adverse effects. mGR, membrane glucocorticoid receptor; PLA2, phospholipase A2; cGR, cytoplasmic glucocorticoid receptor;
GRE, glucocorticoid response element; Hsp70·HOP·Hsp90, multiprotein complex including chaperones such as heat shock proteins and the glucocorticoid receptor;
Hsp90·FKBP52·p23, multiprotein complex including chaperones, co-chaperones, and the glucocorticoid receptor.
FIGURE 2 | Glucocorticoid dosing in induction of remission schemes. High-dose oral glucocorticoid schemes (blue) apply starting doses of oral glucocorticoids at
0.8–1.0 mg/kg/day, with slow tapering, reaching low glucocorticoid doses by 24 weeks of therapy. Recent schemes (green) apply methylprednisolone pulses followed
by medium starting doses of oral glucocorticoids (<0.5 mg/kg/day) with a faster tapering, reaching low glucocorticoid doses by 12 weeks of therapy.
SLE (24–26). An increased incidence of these infections occurs in
patients with kidney disease (
27). Although bacterial infections in
lungs, skin, and urinary tract are far more frequent (25, 28, 29),
the risk for both bacterial and opportunistic infections increases
progressively with the use of medium- to high-dose of GC
(
30–33). The risk of infections associated with high-dose GC
Frontiers in Medicine | www.frontiersin.org 3 February 2021 | Volume 8 | Article 622225

Mejía-Vilet and Ayoub The Use of Glucocorticoids in Lupus Nephritis
TABLE 1 | Reported associations between glucocorticoid (GC) administration and adverse effects.
Methylprednisolone pulses High GC doses Longer time under high GC doses Cumulative GC dose
Acute cardiovascular events Cardiovascular events Cardiovascular events Cardiovascular events
Acute cerebrovascular event Cerebrovascular events Bacterial and opportunistic infection Hypertension
Uncontrolled glucose Insulin resistance Insulin resistance Insulin resistance
Uncontrolled hypertension Cushingoid features Cushingoid features Skin thinning, bruising
Peptic ulcer disease Weight gain Hypertension
Myopathy Dyslipidemia Osteoporosis and vertebral fractures
Mood disorders Glaucoma Sleep disorders
Psychiatric Osteoporosis Avascular necrosis
Sleep disorders
Avascular necrosis
administration seems to be independent of the use of other
immunosuppressive medications (21).
Studies of infections with administration of
methylprednisolone pulses have also been confounded by
indication, due to the traditional administration of this treatment
in combination wit h other aggressive immunosuppressive
regimens to sicker patients (32, 34). Some studies suggest that
the risk of infe c tion is lower with the use of methylprednisolone
pulses of less than 1.5 g in total (35, 36). It has also been
hypothesized that the shorter duration of pulse therapy (3–5
days) may limit the prolonged suppression of T-cell responses,
which usually peaks after 21 days of GC administration (37).
Therefore, methylprednisolone pulses of less than 1.5 g in total
followed by reduced oral GC may potentially decrease the
incidence of steroid induced infections. Additional preventive
measures include vaccination and the use of prophylactic
antibiotics and antivirals when indicated (38, 39).
BONE DISEASE
Avascular bone necrosis occurs in 5–15% of patients with SLE.
It is most commonly found in the femoral head, but may occur
in other weight-bearing joints, and may occur bilaterally (
40–
42). The pathophysiology of avascular bone necrosis is not fully
understood and suggested mechanisms are reviewed elsewhere
(
43). As for infections, avascular bone necrosis has been reported
to occur more frequently in association with lupus nephritis
(44, 45). Also, it has been associated with GC pulse therapy (46),
the peak initial GC dose (47, 48), and the high cumulative GC
doses in the first months of treatment (40, 49).
The prevalence of osteoporosis in SLE is 10 to 20%, with
up to 20% of patients experiencing vertebral fractures (50).
Glucocorticoids increase bone resorption and reduce bone
formation. The former is more pronounced in the first months
of steroid use while the latter becomes predominant with
chronic GC use (51). Osteoporosis and vertebral fractures have
been associated with higher GC doses, cumulative doses, and
prolonged administration (
52). The risk of osteoporotic fractures
has been estimated to increase 4.2% for each 1 mg per day
of prednisone (
19). As bone loss develops over a long-time,
many studies with short follow-up fail to assess the impact of
GC therapy on bone density. Assessment of risk for fractures
and of the need for concomitant preventive therapies including
calcium, vitamin D, and bisphosphonates are recommended for
all patients on GC therapy and are reviewed elsewhere (
53, 54).
Metabolic Disease
Long-term and high-dose GC therapy are associated with
pro-atherogenic disturbances that characterize the metabolic
syndrome (
55). This syndrome occurs in 30 to 40% of patients
with SLE and has been associated with higher disease activity,
past or present history of LN, and higher oral doses of GCs.
Its prevalence varies according to age and ethnicity as expected
(56, 57).
Insulin resistance increases in patients with SLE on oral GC
above 7.5 mg per day (58). Furthermore, the risk of diabetes
increases 2- to 4-fold in non-diabetic patients with SLE, especially
with increasing years of chronic GC use (
59–61). In patients with
pre-existing diabetes, exacerbation of the disease is particularly
severe i n patients with poor glycemic control at baseline (62, 63).
Hypertension is common in SLE and LN patients, with a
prevalence up to 70% when assessed by 24-h blood pressure
monitoring (64). Acute exacerbation of hypertension is frequent
during pulse GC therapy. Although hypertension during an
active LN is mediated by salt-sensitive mechanisms (65), the risk
of hypertension has been also reported to be higher in patients
exposed to GC, and has been associated with the duration of
exposure and the daily dosage of GCs (61, 66, 67).
Glucocorticoids contribute to weight gain by increasing the
appetite for high caloric, high fat food intake (
68, 69). The weight
gain is characterized by central hypertrophy of adipose tissue
with concomitant thinning of peripheral subcutaneous adiposity,
providing a lipodystrophic appearance (Cushingoid phenotype)
(70). Up to 60–70% of patients prescribed long-term GCs report
weight gain (52), and thi s effect has been associated with doses of
GC above 5 mg per day (52, 61).
CARDIOVASCULAR DISEASE
It is known that the incidence of cardiovascular events is
increased in SLE, particularly, in patients with lupus nephritis
and chronic kidney disease (23). Although it is difficult to
Frontiers in Medicine | www.frontiersin.org 4 February 2021 | Volume 8 | Article 622225
Mejía-Vilet and Ayoub The Use of Glucocorticoids in Lupus Nephritis
differentiate the effect of dise ase activity, traditional risk factors,
and tre atment-related factors; the use of medium- to high-
dose GCs has been associated with increased cardiovascular
events, subclinical atherosclerosis such as carotid intima-media
thickening, severity of coronary calcifications, and severity of
arterial stiffness (
71, 72). The risk of cardiovascular events is
estimated to increase 5-fold in SLE patients taking >20 mg
per day of prednisone (23), and 3-fold in those who develop
cushingoid features (73). Cardiovascular events may be reduced
by administering lower peak GC doses, faster GC tapering, and by
limiting cumulative dose. In fact, reductions in cumulative oral
GC were associated with lower incidence of cardiovascular events
in a reported cohort study (74).
STRATEGIES TO MINIMIZE
GLUCOCORTICOID EXPOSURE DURING
THE INDUCTION PHASE OF TREATMENT
The treatment of lupus nephritis has been traditionally divided
into an induction phase of intense immunosuppression, aimed
to quickly suppress inflammation, followed by a prolonged
maintenance phase, directed to consolidate response and to
prevent disease flares (75). For the induction phase, current
guidelines recommend the use of medium to high-dose GCs,
combined with an immunosuppressant such as mycophenolate
mofetil, cyclophosphamide, and more recently, calcineurin
inhibitors (38, 39). Next, we describe strategies aimed to reduce
exposure while keeping treatment efficacy. These strategies
have 3 main objectives: (1) reducing the peak GC dose, (2)
reducing t h e duration of exposure to high-dose GC via a
faster GC tapering, and (3) limiting the cumulative dose from
prolonged administration.
THE USE OF INTRAVENOUS
METHYLPREDNISOLONE PULSES TO
LIMIT GLUCOCORTICOID EXPOSURE
Administration of methylprednisolone pulses may allow the
use of lower initial oral GC doses (lower peak GC dose)
with a faster tapering schedule (lower exposure to high
GC doses). Several clinical studies in lupus nephritis have
included methylprednisolone pulses, followed by moderate (≤0.5
mg/kg/day) doses of oral GCs (Figure 3) (76–78). The Euro
Lupus Nephritis Trial (ELNT) scheme (77) included three
750 mg methylprednisolone pulses, followed by 0.5 mg/kg/day
prednisone slowly tapered to 10 mg/day by 6 months. This
trial reported renal response rates (complete and partial) around
20 and 50% at 6- and 12-months, respectively, and long-term
preser vation of kidney function (77, 79).
The MYLUPUS trial (80) is the only randomized clinical trial
that compared the efficacy of medium-dose oral GC therapy
to high-dose GC. In this trial, all patients received three 0.5 g
methylprednisolone pulses plus extended-release mycophenolate
acid. Subjects were randomized to either high-dose oral GC
scheme (starting dose 1 mg/kg/day) or to a reduced-dose oral
GC scheme (starting dose ≈0.5 mg/kg/day). Complete and tot a l
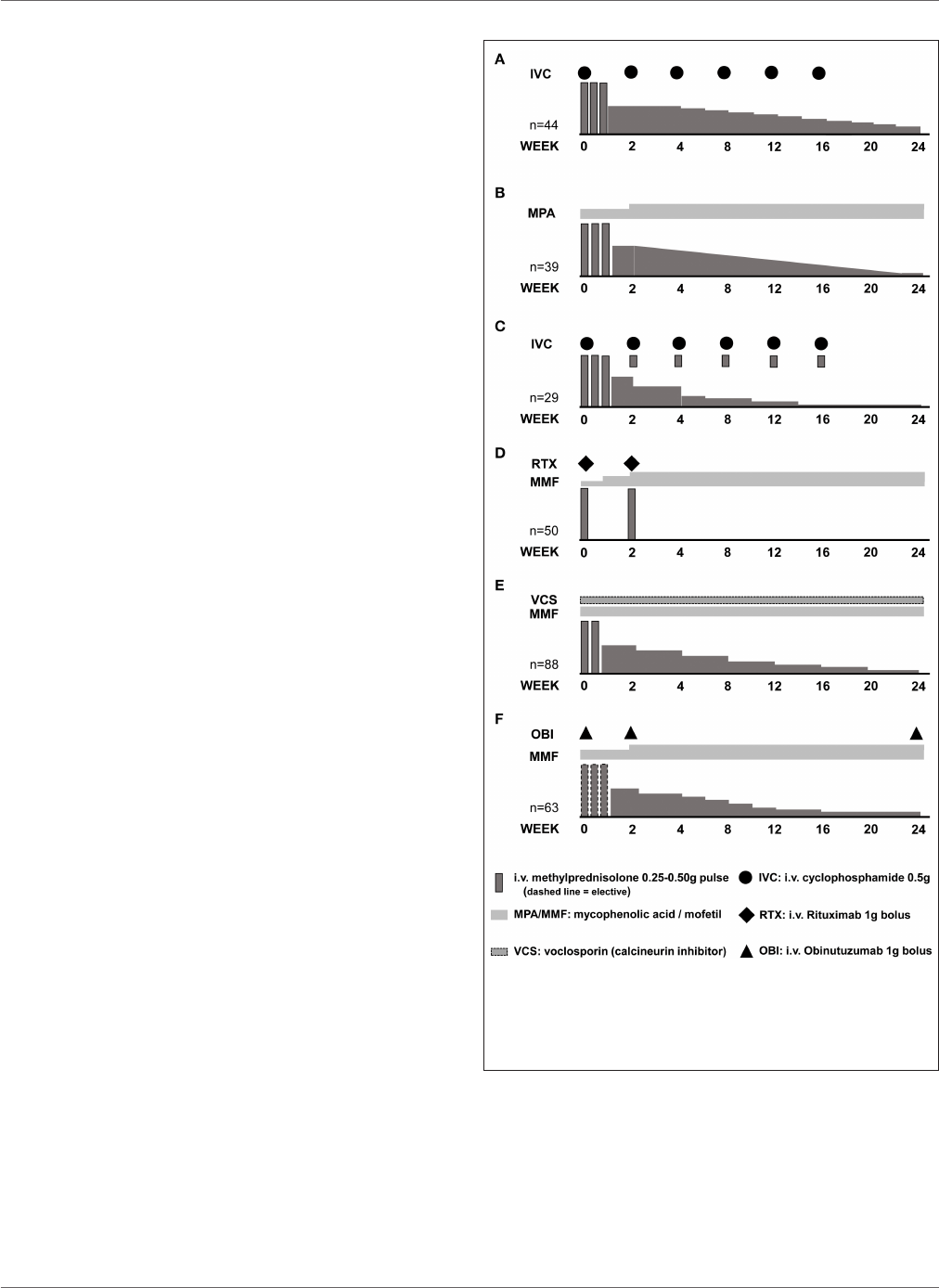
FIGURE 3 | Induction of remission schemes in several studies. (A) Euro Lupus
Nephritis Trial low-dose cyclophosphamide arm; (B) MYLUPUS reduced-dose
glucocorticoid arm; (C) Lupus-Cruces protocol; (D) RITUXILUP protocol; (E)
AURA-LV study voclosporin-treated arm; (F) NOBILITY study
obinutuzumab-treated arm.
response rates were similar at 6 months, 19 vs. 21% and 67 vs.
56%, respecti vely, in both groups.
In a trial evaluating a combination of calcineurin
inhibitor, mycophenolate mofetil and GCs vs. intravenous
cyclophosphamide, all patients received three 0.5 g/day
methylprednisolone pulses followed by 0.6 mg/kg oral
Frontiers in Medicine | www.frontiersin.org 5 February 2021 | Volume 8 | Article 622225

Mejía-Vilet and Ayoub The Use of Glucocorticoids in Lupus Nephritis
prednisone slowly tapered to 10 mg/d a y by week 16. Response
rates of 84 and 63% at 6-months, were documented in the multi-
targeted therapy and cyclophosphamide groups, respectively,
and of 78% in both groups by 2 years of therapy (81–83).
More recent clinical trials have used lower doses of
methylprednisolone pulses combined with a lower and faster
oral GC tapering. In the AURA-LV (
84) and AURORA
(NCT03021499) trials evaluating combination therapy of
voclosporin (a novel calcineurin inhibitor) with mycophenolate
mofetil and oral GC, patients were treated with two 0.25–0.5 g
methylprednisolone pulses, followed by a fixed 20–25 mg/d
starting oral prednisone rapidly tapered to 5 mg by 12 weeks.
Among clinical trials in LN, these two trials used the lowest peak
oral GC doses and the faster tapering (Table 2). At 12 months,
complete and total renal response rates of 49 vs. 24%, and 67 vs.
48%, respectively, were observed in the multi-targeted treatment
and control groups in the AURA-LV trial (
84).
Uncontrolled single center experiences also suggest that
treatment with methylprednisolone pulses allows a safe
administration of lower starting oral GCs, and a faster tapering
without compromising response and possibly reducing adverse
effects. For example, the “Lupus Cruces” protocol for class III or
IV LN includes the administration of three methylprednisolone
pulses between 0.25–0.50 g, and an extra pulse of 0.1 g along with
each cyclophosphamide bolus, following t he ELNT scheme. The
starting oral GC in this protocol was below 30 mg per day. In two
reports, including 15 and 29 patients, response rates of 60 and
80%, and 86 and 87%, have been achieved at 6- and 12-months,
respectively, with relapse rates below 15%. More importantly,
the incidence of GC-related adverse effects was reduced to
7%, a significantly lower percentage when compared to that of
historical or concurrent cohorts treated with higher doses of oral
GC (48, 90).
Therefore, as evidenced in clinical trials and single-center
experiences, the use of methylprednisolone pulses may allow
reducing the starting oral GC doses and the duration of the
exposure to high GC doses by allowing a faster tapering.
THE USE OF “REDUCED-DOSE”
INTRAVENOUS METHYLPREDNISOLONE
PULSES
There are no specific reports evaluating the dose of
methylprednisolone in lupus nephritis. Although the use of
methylprednisolone pulses has been associated with higher
risk of infection in some cohort studies (
32), these studies
do not control for methylprednisolone dose. A small clinical
trial including 21 patients with SLE (6 of them with nephritis)
suggested that clinic a l outcomes are similar when using three
daily 100 mg vs. 1 g methylprednisolone pulses. However, this
trial did not control for other important variables such as
concomitant treatment (91). While quality evidence is still
low to support lower doses of meth ylprednisolone pulses,
pharmacologic studies suggest that pulse doses above 0.5 g
provide little additional anti-inflammatory benefit, and as
mentioned earlier, may be associated with a higher incidence of
adverse effects.
MEDIUM-DOSE GLUCOCORTICOID IN
COMBINATION WITH NEW
IMMUNOSUPPRESSANT DRUGS AND
BIOLOGICS
Combination therapy of mycophenolate mofetil, calcineurin
inhibitors, and glucocorticoids may facilitate the use of lower
GC doses. As previously mentioned, the AURA-LV trial used
a forced reduced steroid taper along with mycophenolate ±
voclosporin. The multi-targeted group showed 67% response rate
by 12 months of treat ment (
84).
A recent trial evaluated the combination t herapy of
obinutuzumab, a novel B-cell depleting t herapy , with
mycophenolic acid analogs. All patients received a starting
oral prednisone dose of 0.5 mg/kg with a fast taper to 7.5 mg by
week 12, and optional methylprednisolone pulses. This trial has
reported 52-week CR rates of 35%, maintained at 40 and 41% by
76 and 104 weeks, respectively (89).
Other immunosuppressants such as Janus kinase inhibitors,
spleen tyrosine kinase inhibitors and biologics such as
anifrolumab and ustekinumab, are being tested in patients
with LN. Their addition might also facilitate the use of lower
dose glucocorticoids in the future.
SCHEMES FREE OF ORAL
GLUCOCORTICOIDS
After initial reports describing the potentia l use of rituximab
without increasing GC dose in renal (
92) and non-renal lupus
(93), the UK group from the Imperial College in London
reported their first 50 patient experience with the RITUXILUP
scheme (86). This regimen consists of 2 doses of rituximab
1 g administered with 0.5 g met h ylprednisolone followed by
mycophenolate mofetil and no oral glucocorticoids. The initial
report, which included class III, IV, and V LN patients, showed
6-month complete and total response rates of 32 and 62%,
respectively. During follow-up, kidney function was preserved
in most patients, with 22% of patients experiencing nephrotic
relapses. Importantly, unlike the LUNAR tria l (94) that failed
to demonstrate a benefit of added rituximab to the standard of
care therapy, depletion of B-cells to <5 B lymphocytes/mL was
achieved in 93% of patients. The importance of B-cell depletion is
supported by a sub-analysis from the LUNAR trial showing that
complete response was more frequent in t h ose subjects with B
cell depletion (95). Therefore, although not yet demonstrated in
a clinical trial, the RITUXILUP scheme supports the concept that
the use of biologic drugs may facilitate the administration of GC
free regimen in some patients with LN.
Targeting the activated complement system with complement
inhibitors may also promote GC-reduced or GC-free regimens.
Although complement inhibition in lupus nephritis has been
used in a few case reports (
96, 97), particularly in the context
of concomitant thrombotic microangiopathy, the CLEAR (
98)
Frontiers in Medicine | www.frontiersin.org 6 February 2021 | Volume 8 | Article 622225

Mejía-Vilet and Ayoub The Use of Glucocorticoids in Lupus Nephritis
TABLE 2 | Estimated cumulative glucocorticoid doses in a 24-week period for a 60 kg patient in different induction to remission schemes.
Regimen Methylprednisolone
total cumulative
dose (g)
Oral prednisone
total cumulative
dose (g)
Oral prednisone
average dose
(mg/day)
Total GC dose (g)
Modified NIH, 2001 (76) 9.00 2.84 16.9 11.8
ELNT, 2002 (
77) 2.25 3.12 18.5 5.37
ALMS, 2009 (
85) – 4.27 25.4 4.27
MYLUPUS, 2011 (
80) 1.50 2.14 12.7 3.64
RITUXILUP, 2013 (
86) 1.00 – – 1.00
LupusCRUCES, 2014 (
48) 1.50-3.00 1.30-1.50 8.0-9.0 2.80–4.50
Chinese multitarget, 2015 (
81) 1.5 3.25 16.2 4.75
4+2 Rituximab, 2015 (
87) 2.70 2.52 15.0 5.22
AURA-LV, 2019 (
84) 1.00 1.33 7.9 2.33
BLISS-LN, 2020 (
88) 0.50–3.00* 3.12–4.27 18.5–25.4 3.12–4.27
NOBILITY, 2020 (
89) 0.75–3.00* 1.79–1.93 10.6–11.5 1.79–1.93
*
Methylprednisolone pulses elective at discretion of the investigator.
and ADVOCATE (
99) studies in ANCA-associated vasculitis
suggest this may be an approach worth investigating in lupus
nephritis. In these studies, administration of avacopan (an oral
complement C5aR inhibitor) along with cyclophosphamide or
rituximab, allowed the administration of a GC-free re gimen with
higher remission rates at 52 weeks of follow-up in patients with
ANCA-associated vasculitis (99).
CONCOMITANT USE OF ANTIMALARIALS
The use of anti malarial in all patients with SLE and lupus
nephritis is recommended in recent guidelines (
38, 39). Although
unexplored in controlled trials, combination schemes with
antimalarial may add to the use of lower doses of GC by an
enhanced effect for remission (
100–102). O ther demonstrated
benefits from antimalarial, as the protective effe ct for damage
accrual (103, 104), infections (33), and mortality (105), may add
to th e potential benefit of GC-reduced regimens.
GLUCOCORTICOIDS DURING THE
MAINTENANCE PHASE OF TREATMENT
Maintenance therapy in lupus nephritis a ims to consolidate
the response obtained after the induction phase of t h erapy,
and to prevent systemic and renal relapses. Current guidelines
suggest tapering glucocorticoids to “the lowest possible dose”
and to consider discontinuation after 1 2 months of complete
remission (
39).
Although there is no solid evidence in lupus nephritis, t h e
CORTICOLUP trial (106) evaluated discontinuation of steroid
in stable SLE patients (34–41% had history of LN). In this trial,
patients receiving 5 mg of prednisone who have been stable for
1 year (the median quiescence duration was ≈5 yea rs) were
randomized to suspend or continue prednisone at the same
dose. Disease flares were observed in 27% of patients who
suspended prednisone vs. 7% in those who continued prednisone
at 5 mg per day (RR 0.2, 0.01–0.7, p = 0 .003) . Only 3 patients
had renal flares and the study was underpowered to evaluate
the subgroup of patients with LN. Noteworthy, there were no
differences in adverse events or damage accrual in both groups
measured using t h e glucocorticoid toxicity index (
107) and the
SDI, respe ctively .
This study suggests that a low-dose of glucocorticoids at
5 mg per day may be safe and keeps patients free from disease
flares. In other studies, the longer duration of the GC therapy
before suspension has also been associated with less disease
flares (108). A recent EULAR expert consensus suggested
that at ≤5 mg/day, there is a low level of harm related to
GC’s main adverse effects (109), however, acknowledges that
the actual risk of harm is patient-specific. Therefore, long-
term glucocorticoid therapy must be balanced individually
considering individual risk factors for flares (e.g., partial instead
of complete response, persistently low C3), against individual
risk factors for GC related adverse effects (e.g., age, cumulative
GC dose, cardiovascular risk factors, presence of metabolic
disease, etc.).
STRATEGIES TO MINIMIZE
CORTICOSTEROIDS DURING THE
MAINTENANCE PHASE
Antimalarial Treatment
Antimalarials have been associated with lower incidence of
disease flares in several observational cohort studies (
110,
111). Moreover, reports of successful with drawal of therapy
in SLE patients have repeatedly found antimalarial treatment
and duration of remission as the main factors associated
with decreased odds of flares (
112, 113). Also, as pre viously
mentioned, antimalarials may reduce long-term damage from the
disease activity (
114).
Frontiers in Medicine | www.frontiersin.org 7 February 2021 | Volume 8 | Article 622225

Mejía-Vilet and Ayoub The Use of Glucocorticoids in Lupus Nephritis
BIOLOGICS FOR MAINTENANCE
THERAPY
Although evidence is still scarce, there is growing data suggesting
that the use of cert a in biologics during the maintenance phase
may aid in achieving sustained remission. For patients already
on glucocorticoids, the RITUXIRESCUE regimen includes the
administration of rituximab and methylprednisolone without
increasing oral GC dose. This regimen showed a response rate
of 78% in LN relapses, furthermore it allowed reduction or
discontinuation of oral GC in more than 50% of patients during
follow up (
92).
An Italian strategy consisting of four 375 g/m
2
rituximab
doses reinforced by two additional doses at 1 and 2 months after
(the 4+2 rituximab scheme), showed no flares during follow-
up without the need for additional maintenance therapy beyond
5 mg of prednisone per day (87). Other small reports have
highlighted the potential role of rituximab as a maintenance drug
allowing glucocorticoid suspension (115). Therefore, although
rituximab has not been tested for maintenance in a clinical trial,
its use may aid in preventing flares during GC withdrawal.
In the BLISS-LN trial, the addition of belimumab to standard
of care therapy (MMF or cyclophosphamide plus GC) showed
a better response and a stable glomerular filtration rate beyond
the induction phase, for up to 2 years of follow up (
88).
Furthermore, t here have been small reports (116–118) suggesting
that belimumab therapy may allow reduction or suspension of
maintenance GCs, but this remains to be further st udied.
Likewise, the NOBILITY trial has reported that the addition
of obinutuzumab to standard of care therapy favored a sustained
response, better glomerular filtration rate, and better serological
profile at 76 weeks and onwards (
89). This suggests that B cell
targeted therapy may potentially facilitate GC withdrawal or at
least a safe reduction to <5 mg/d of prednisone.
FUTURE STEPS AND A WORD OF
CAUTION
Although recent advances in drug development in lupus nephritis
promote the use of lower glucocorticoid doses, we must
acknowledge that “one size does not fit all” patients. For example,
patients with severe lupus nephritis presenting with a glomerular
filtration rate below 30 mL/min/1.73 m
2
have been excluded
from most clinical trials, and there are no data to support the
effectiveness of reduced glucocorticoid doses in this group of
patients. Moreover, many of the published studies are single-
center and observational reports subject to bias. Therefore,
caution and case-by-case evaluation is recommended in selecting
an appropriate glucocorticoid therapy.
Future studies in lupus nephritis will likely aim at using
the lowest effective dose of glucocorticoids or glucocorticoid-
free regimens. Studying the safety and efficacy of calcineurin
inhibitors, biologic drugs or perhaps complement inhibitors
in combination with standard of care therapy might lead
successfully to this aim.
CONCLUSIONS
The anti-inflammatory properties of GC have always been
counterbalanced by their side effects. Adverse effects may be
associated with peak doses, time under high doses, or cumulative
doses. An objective for current and future management of lupus
nephritis is to develop strate gies that increase response to t h erapy
with the least glucocorticoid exposure.
AUTHOR CONTRIBUTIONS
JM-V and IA designed the concept, planned, and performed
this work.
REFERENCES
1. Hench PS, Kendall EC, Slocumb CH, Polley HF. Adrenocortical
hormone in arthritis : preliminary report. Ann Rheum Dis. (1949)
8:97–104. doi: 10.1136/ard.8.2.97
2. Burns CM. The history of cortisone discovery and development. Rheum Dis
Clin North Am. (2016) 42:1–14. doi: 10.1016/j.rdc.2015.08.001
3. Cameron JS. A Comparison of cortisone and prednisone in
treatment of rheumatoid arthritis. Br Med J. (1957) 2:199–
202. doi: 10.1136/bmj.2.5038.199
4. Cameron JS. Lupus nephritis: an historical perspective 1968-1998. J Nephrol.
(1999) 12(Suppl. 2):S29–41.
5. Pollak VE, Pirani CL, Schwartz FD. The natural history of the renal
manifestations of systemic lupus erythematosus. 1964. J Am Soc Nephrol.
(1997) 8:1189–98.
6. Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids —
New mechanisms for old drugs. N Engl J Med. (2005) 353:1711–
23. doi: 10.1056/NEJMra050541
7. Buttgereit F, Wehling M, Burmester G-R. A new hypothesis of
modular glucocorticoid actions: steroid treatment of rheumatic diseases
revisited. Arthritis Rheum. (1998) 41:761–73. doi: 10.1002/1529-
0131(199805)41:5<761::AID-ART2>3.0.CO;2-M
8. Stahn C, Butt g ereit F. Genomic and nongenomic effects of glucocorticoids.
Nat Clin Pract Rheumatol. (2008) 4:525–33 doi: 10.1038/ncprheum0898
9. Strehl C, Buttgereit F. Unraveling the functions of the membrane-bound
glucocorticoid receptors: first clues on origin and functional activity. Ann
N Y Acad Sci. (20 14 ) 1318:1–6. doi: 10.1111/nyas.12364
10. Buttgereit F. Standardised nomenclature for glucocorticoid dosages
and glucocorticoid treatment regimens: current questions and
tentative answers in rheumatology. Ann Rheum Dis. (2002)
61:718–22. doi: 10.1136/ard.61.8.718
11. Buttgereit F, Straub RH, Wehling M, Burmester GR. Glucocorticoids
in the treatment of rheumatic diseases: an update on the mechanisms
of action. Arthritis Rheum. (2004) 50:3 40 8– 17 . doi: 10.1002/art.
20583
12. Schmid D, Burmester GR, Tripmacher R, Kuhnke A, Buttgereit F.
Bioenergetics of human peripheral blood mononuclear cell metabolism in
quiescent, activated, and glucocorticoid-treated states. Biosci Rep. (2000)
20:289–302. doi: 10.1023/A:1026445108136
13. Williams DM. Clinical pharmacology of corticosteroids. Respir Care. (2018)
63:655–70. doi: 10.4187/respcare.06314
14. Scherholz ML, Schlesinger N, Androulakis IP. Chronopharmacology
of glucocorticoids. Adv Drug Deliv Rev. (2019) 151–2:245–
261. doi: 10.1016/j.addr.2019.02.004
15. Zen M, Iaccarino L, Gatto M, Bettio S, Nalotto L, Ghirardello
A, et al. Prolonged remission in Caucasian patients with SLE:
prevalence and outcomes. Ann Rheum Dis. (2015) 74:2117–
22. doi: 10.1136/annrheumdis-2015-207347
Frontiers in Medicine | www.frontiersin.org 8 February 2021 | Volume 8 | Article 622225

Mejía-Vilet and Ayoub The Use of Glucocorticoids in Lupus Nephritis
16. Nossent J, Kiss E, Rozman B, Pokorny G, Vlachoyiannopoulos P, Olesinska
M, et al. Disease activity and damage accrual during the early disease
course in a multinational inception cohort of patients with systemic
lupus erythematosus. Lupus. (2010) 19:949–56. doi: 10.1177/09612033103
66572
17. Gladman DD, Urowitz MB, Rahman P, Ibañe z D, Tam LS. Accrual of
organ damage over time in patients with systemic lupus erythematosus. J
Rheumatol. (2003) 30:1955–9.
18. Karlson EW, Daltroy LH, Lew RA, Wright EA, Partridge AJ, Fossel AH, et al.
The relationship of socioeconomic status, race, and modifiable risk factors
to outcomes in patients with systemic lupus erythematosus. Arthritis Rheum.
(1997) 40:47–56. doi: 10.1002/art.1780400108
19. Al Sawah S , Zhang X, Zhu B, Magder LS, Foster SA, Iikuni N, et al. Effect of
corticosteroid use by dose on the risk of developing organ damage over time
in systemic lupus erythematosus–the Hopkins Lupus Cohort. Lupus Sci Med.
(2015) 2:e000066. doi: 10.1136/lupus-2014-00 00 66
20. Zen M, Iaccarino L, Gatto M, Bettio S, Saccon F, Ghirardello A, et al. The
effect of different durations of remission on damage accrual: results from
a prospective monocentric cohort of Cauc asian patients. Ann Rheum Dis.
(2017) 76:562–5. doi: 10.1136/annrheumdis-2016-210154
21. Singh JA, Hossain A, Kotb A, Wells G. Risk of serious infections
with immunosuppressive drugs and glucocorticoids for lupus nephritis:
a systematic review and network meta-analysis. BMC Med. (2016)
14:137. doi: 10.1186/s12916-016-0673-8
22. Thamer M, Hernan MA, Zhang Y, Cotter D, Petri M. Prednisone, lupus
activity, and permanent organ damage. J Rheumatol. (2009) 36 :56 0–
4. doi: 10.3899/jrheum.080828
23. Magder LS, Petri M. Incidence of and risk factors for adverse cardiovascular
events among patients with systemic lupus erythematosus. Am J Epidemiol.
(2012) 176:708–19. doi: 10.1093/aje/kws130
24. Ward MM, Pyun E, Studenski S. Causes of death in systemic lupus
erythematosus, long-term follow up of an inception cohort. Arthritis Rheum.
(1995) 38:1492–9. doi: 10.1002/art.1780381016
25. Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A,
Lavilla P, et al. Morbidity and mortality in systemic lupus
erythematosus during a 10-year period. Medicine. (2003)
82:299–308. doi: 10.1097/01.md.0000091181.93122.55
26. Yap DYH, Tang CSO, Ma MKM, Lam MF, Chan TM. Survival analysis and
causes of mortality in patients with lupus nephritis. Nephrol Dial Transplant.
(2012) 27:3248–54. doi: 10.1093/ndt/gfs073
27. Feldman CH, Hiraki LT, Winkelmayer WC, Marty FM, Franklin JM ,
Kim SC, et al. Serious infections among adult medicaid beneficiaries with
systemic lupus erythematosus and lupus nephritis. Arthritis Rheumatol.
(2015) 67:1577–85. doi: 10.1002/art.39070
28. Gladman DD, Hussain F, Iban D, Urowitz MB. The nature and outcome
of infection in systemic lupus erythematosus. Lupus. (2002) 11:234–
9. doi: 10.1191/0961203302lu170oa
29. Noel V. Risk factors and prognostic influence of infection in a single cohort
of 87 adults with systemic lupus erythematosus. Ann Rheum Dis. (2001)
60:1141–4. doi: 10.1136/ard.60.12.1141
30. Ruiz-Irastorza G, Olivares N, Ruiz-Arruza I, Martinez-Berriotxoa A,
Egurbide M-V, Aguirre C. Predictors of major infections in systemic
lupus erythematosus. Arthritis Res Ther. (2009) 11:R109. doi: 10.1186/a
r2764
31. Rúa-Figueroa I, López-Longo FJ, Del Campo V, Galindo-Izquierdo
M, Uriarte E, Torre-Cisneros J, et al. Bacteremia in systemic lupus
erythematosus in patients from a Spanish Registry: risk factors, clinical
and microbiological characteristics, and outcomes. J Rheumatol. (2020)
47:234–40. doi: 10.3899/jrheum.180882
32. Pimentel-Quiroz VR, Ugarte-Gil MF, Harvey G, Wojdyla D, Pons-Estel
GJ, Quintana R, et al. Factors predictive of serious infections over
time in systemic lupus erythematosus patients: data from a multi-ethnic,
multi-national, Latin American lupus cohort. Lupus. (2019) 28:1101–
10. doi: 10.1177/0961203319860579
33. González-Echavarri C, Capdevila O, Espinosa G, Suárez S, Marín-Ballvé A,
González-León R, et al. Infections in newly diagnosed Spanish patients with
systemic lupus erythematosus: data from the RELES cohort. Lupus. (2018)
27:2253–61. doi: 10.1177/0961203318811598
34. Teh CL, Wan SA, Ling GR. Severe infections in systemic lupus
erythematosus: disease pattern and predictors of infection-related mortality.
Clin Rheumatol. (2018) 37:2081–6. doi: 10.1007/s10067- 01 8 -4 10 2- 6
35. Badsha H, Kong KO, Lian TY, Chan SP, Edwards CJ, Chng HH. Low-
dose pulse methylprednisolone for systemic lupus erythematosus flares is
efficacious and has a decreased risk of infectious complications. Lupus.
(2002) 11:508–13. doi: 10.1191/0961203 30 2lu 24 3oa
36. Badsha H, Edwards CJ. Intravenous pulses of methylprednisolone for
systemic lupus erythematosus. Semin Arthritis Rheum. (2003) 32:370–
7. doi: 10.1053/sarh.2002.50003
37. Cutolo M, Seriolo B, Pizzorni C, Secchi ME, Soldano S, Paolino S, et
al. Use of glucocorticoids and risk of infections. Autoimmun Rev. (2008)
8:153–5. doi: 10.1016/j.autrev.2008.07.010
38. Fanouriakis A, Kostopoulou M, Cheema K, Anders HJ, Aringer M,
Bajema I, et al. 2019 Update of the j oint European League Against
Rheumatism and European Renal Association-European Dialysis and
Transplant Association (EULAR/ERA-EDTA) recommend ations for the
management of lupus nephritis. Ann Rheum Dis. (2020) 79:713–
23. doi: 10.1136/annrheumdis-2020-eular.3936
39. KDIGO: Kidney Disease Improving Global Outcomes Clinical Practice
Guideline for Glomerulonephritis. Kidney Int. (2021). Available online at:
kdigo.org/guidelines/
40. Petri M. Musculoskeletal complications of systemic lupus erythematosus
in the Hopkins lupus cohort: an update. Arthritis Care Res. (1995) 8:137–
45. doi: 10.1002/art.1790080305
41. Gladman DD, Dhillon N, Su J, Urowitz MB. Osteonecrosis in SLE:
prevalence, patterns, outcomes and predictors. Lupus. (2018) 27:76–
81. doi: 10.1177/0961203317711012
42. Sayarlioglu M, Yuzbasioglu N, Inanc M, Kamali S, Cefle A,
Karaman O, et al. Risk factors for avascular bone necrosis in
patients with systemic lupus erythematosus. Rheumatol Int. (2012)
32:177–82. doi: 10.1007/s00296-010-1597-9
43. Shah KN, Racine J, Jones LC, Aaron RK. Pathophysiology and risk
factors for osteonecrosis. Curr Rev Musculoskelet Med. (2015) 8:201–
9. doi: 10.1007/s12178-015-9277-8
44. Hussein S, Suitner M, Béland-Bonenfant S, Baril-Dionne A, Vandermeer
B, Santesso N, et al. Monitoring of osteonecrosis in systemic lupus
erythematosus: a systematic review and metaanalysis. J Rheumatol. (2018)
45:1462–76. doi: 10.3899/jrheum.170837
45. Mok CC, Lau CS, Wong RWS. Risk factors for avascular bone
necrosis in systemic lupus erythematosus. Rheumatology. (1998) 37:895–
900. doi: 10.1093/rheumatology/37.8.895
46. Mosca M, Tani C, Carli L, Bombardieri S. Glucocorticoids in systemic lupus
erythematosus. Clin Exp Rheumatol. (2011) 29:S126–9.
47. Sciascia S, Mompean E, Radin M, Roccatello D, Cuadrado MJ. Rate of
adverse effects of medium- to high-dose glucocorticoid therapy in systemic
lupus erythematosus: a systematic review of randomized control trials. Clin
Drug Investig. (2017) 37:519–24. doi: 10.1007/s40261-017-0518-z
48. Ruiz-Irastorza G, Danza A, Perales I, Villar I, Garcia M, Delgado S, et al.
Prednisone in lupus nephritis: how much is enough? Autoimmun Rev. (2014)
13:206–14. doi: 10.1016/j.autrev.2013.10.013
49. Chen HL, Shen LJ, Hsu PN, Shen CY, Hall SA, Hsiao FY. Cumulative burden
of glucocorticoid-related adverse events in patients with systemic lupus
erythematosus: findings from a 12-year longitudinal study. J Rheumatol.
(2018) 45:83–9. doi: 10.3899/jrheum.160214
50. Bultink IEM, Lems WF, Kostense PJ, Dijkmans BAC, Voskuyl AE. Prevalence
of and risk factors for low bone mineral density and vertebral fractures
in patients with systemic lupus erythematosus. Arthritis Rheum. (2005)
52:2044–50. doi: 10.1002/art.21110
51. Adami G, Saag KG. Glucocorticoid-induced osteoporosis:
2019 concise clinical re view. Osteoporos Int. (2019) 30:1145–
56. doi: 10.1007/s00198-019-04906-x
52. Curtis JR, Westfall AO, Allison J, Bijlsma JW, Freeman A, George V, et
al. Population-based assessment of adverse events associated with long-
term glucocorticoid use. Arthritis Rheum. (2006) 55:420–6. doi: 10.100 2/art.
21984
53. Buckley L, Guyatt G, Fink HA, Cannon M, Grossman J, Hansen KE, et al.
2017 American College of Rheumatology guideline for the prevention and
Frontiers in Medicine | www.frontiersin.org 9 February 2021 | Volume 8 | Article 622225

Mejía-Vilet and Ayoub The Use of Glucocorticoids in Lupus Nephritis
treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res. (2017)
69:1095–110. doi: 10.1002/acr.23279
54. Adams J, Wilson N, Hurkmans E, Bakkers M, B alážová P, Baxter M, et al.
2019 EULAR points to consider for non-physician health professionals to
prevent and manage fragility fractures in adults 50 years or older. Ann Rheum
Dis. (2021) 80:57–64. doi: 10.1136/annrheumdis-2020-216931
55. Parker B, Bruce IN. The metabolic syndrome in systemic
lupus erythematosus. Rheum Dis Clin North Am. (2010)
36:81–97. doi: 10.1016/j.rdc.2009.12.004
56. Parker B, Urowitz MB, Gladman DD, Lunt M, Bae SC, Sanchez-Guerrero
J, et al. Clinical associations of the metabolic syndrome in systemic lupus
erythematosus: data from an international inception cohort. Ann Rheum Dis.
(2013) 72:1308–14. doi: 10.1136/annrheumdis-2012-202106
57. Parker B, Urowitz M B, Gladman DD, Lunt M, Donn R , B ae SC, et al.
Impact of early disease factors on metabolic syndrome in systemic lupus
erythematosus: data from an international inception cohort. Ann Rheum Dis.
(2015) 74:1530–6. doi: 10.1136/annrheumdis-2013-203933
58. Sabio JM, Vargas-Hitos JA, Navarrete N, Hidalgo-Tenorio C, Jiménez-
Alonso J, Grupo Lupus Virgen de las Nieves. Effects of low or medium-
dose of prednisone on insulin resistance in patients with systemic lupus
erythematosus. Clin Exp Rheumatol. (2010) 28:483–9.
59. Gulliford MC, Charlton J, Latinovic R. Risk of diabetes associated with
prescribed glucocorticoids in a large population. Diabetes Care. (2006)
29:2728–9. doi: 10.2337/dc06-1499
60. Burt MG, Willenberg VM, Petersons CJ, Smith MD, Ahem MJ, Stranks
SN. Screening for diabetes in patients with inflammatory rheumatological
disease administered long-term prednisolone: a cross-se ctional study.
Rheumatology. (2012) 51:1112–9. doi: 10.1093/rheumatology/kes003
61. Huscher D, Thiele K, Gromnica-Ihle, E, Hein G, Demary W, Dreher R, et
al. Dose-related patterns of glucocorticoid-induced side effects. Ann Rheum
Dis. (2009) 68:1119–24. doi: 10.1136/ard.2008.092163
62. Reynolds RM, Labad J, Sears AV, Williamson RM, Strachan MW, Deary IJ, et
al. Glucocorticoid treatment and impaired mood, memory and metabolism
in people with diabetes: the Edinburgh Type 2 Diabetes Study. Eur J
Endocrinol. (2012) 166:861–8. doi: 10.1530/EJE-12-0041
63. Feldman-Billard S, Lissak B, Kassaei R, Benrabah R, Héron
E. Short-term tolerance of pulse methylprednisolone therapy
in patients with diabetes mellitus. Ophthalmology. (2005)
112:511–5. doi: 10.1016/j.ophtha.2004.10.032
64. Mejia-Vilet JM, López-Hernández YJ, Trujeque-Matos M, Santander-
Velez JI, Cano-Verduz co ML, Cruz C, et al. High frequency
of nocturnal hypertension in lupus nephritis: should ABPM
be implemented in usual practice? Clin Rheumatol. (2020)
39:1147–55. doi: 10.1007/s10067-019-04830-9
65. Mathis KW, Venegas-Pont M, Masterson CW, Wasson KL, Ryan MJ.
Blood pressure in a hypertensive mouse model of SLE is not salt-
sensitive. Am J Phys Regul Integr Comp Phys. (2011 ) 301:R1281–
5. doi: 10.1152/ajpregu.00386.2011
66. Conn HO, Poynard T. Corticosteroids and peptic ulcer: meta-analysis
of adverse events during steroid therapy. J Intern Med. (1994) 236:619–
32. doi: 10.1111/j.1365-2796.1994.tb00855.x
67. Panoulas VF, Douglas KKMJ, Stavropoulos-Kalinoglou A, Metsios
GS, Nig htingale P, Kita MD, et al. Long-term exposure to
medium-dose glucocorticoid therapy associates with hypertension
in patients with rheumatoid arthritis. Rheumatology. (2008)
47:72–5. doi: 10.1093/rheumatology/kem311
68. Strack AM, Sebastian RJ, Schwartz MW, Dalllman MF. Glucocorticoids
and insulin: reciprocal signals for energy balance. Am J Physiol. (1995)
268:R142–9 doi: 10.1152/ajpregu.1995.268.1.R142
69. Dallman MF, la Fleur SE, Pecoraro NC, Gomez F, Houshyar H,
Akana SF. Minireview: glucocorticoids- food intake, abdominal
obesity, and wealthy nations in 2004. Endocrinology. (2 00 4)
145:2633–8. doi: 10.1210/en.2004-0037
70. Fardet L, Cabane J, Lebbé C, Morel P, Flahault A. Incidence and risk factors
for corticosteroid-induced lipodystrophy: a prospective study. J Am Acad
Dermatol. (2 00 7) 57:604–9. doi: 10.1016/j.jaad.2007.04.018
71. Tselios K, Sheane BJ, Gladman DD, Urowitz MB. Optimal
monitoring for coronary he art disease risk in patients with systemic
lupus erythematosus: a systematic review. J Rheumatol. (2016)
43:54–65. doi: 10.3899/jrheum.150460
72. Wu GC, Liu HR, Leng RX, Li XP, Li XM, Pan HF, et al. Subclinical
atherosclerosis in patients with systemic lupus erythematosus:
a systemic review and meta-analysis. Autoimmun Rev. (2015)
15:22–37. doi: 10.1016/j.autrev.2015.10.002
73. Fardet L, Petersen I, Nazareth I. Risk of cardiovascular events in people
prescribed glucocorticoids with iatrogenic Cushing’s syndrome: cohort
study. BMJ. (2012) 345:e4928. doi: 10.1136/bmj.e4928
74. Ruiz-Arruza I, Lozano J, Cabezas-Rodriguez I, Medina JA, Ugarte A,
Erdozain JG, et al. Restrictive use of oral glucocorticoids in systemic lupus
erythematosus and prevention of damage without worsening long-term
disease control: an observational study. Arthritis Care Res. (2018) 70:582–
91. doi: 10.1002/acr.23322
75. Mejia-Vilet JM, Rovin BH. Chapter 59. Epidemiology and Management of
Lupus Nephritis. In: Wallace Daniel J and Hahn Bevra H, editors. Dubois
Systemic Lupus Erythematosus. Philadelphia, PA: Elsevier. (2019). p. 727–44.
doi: 10.1016/B978-0-323-47927-1.00059-1
76. Illei GG, Austin HA, Crane M, Collins L, Gourley MF, Yarb oro CH,
et al. Combination therapy with pulse cyclophosphamide plus pulse
methylprednisolone improves long-term renal outcome without adding
toxicity in patients with lupus nephritis. Ann Intern Med. (2001)
135:248. doi: 10.7326/0003-4819-135-4-2001082 10 -0 00 0 9
77. Houssiau FA, Vasconcelos C, D’Cruz D, Sebastiani GD, Garrido E de R,
Danieli MG, et al. Immunosuppressive therapy in lupus nephritis: the
Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-
dose intravenous cyclophosphamide. Arthritis Rheum. (2002) 46:2121–
31. doi: 10.1002/art.10461
78. Yee CS. EULAR randomised controlled trial of pulse cyclophosphamide and
methylprednisolone versus continuous cyclophosphamide and prednisolone
followed by azathioprine and prednisolone in lupus nephritis. Ann Rheum
Dis. (2004) 63:525–9. doi: 10.1136/ard.2002.003574
79. Houssiau FA, Vasconcelos C, D’Cruz D, Sebastiani GD, de Ramon
Garrido E, Danieli MG, et al. The 10-year follow-up data of the Euro-
Lupus Nephritis Trial comparing low-dose and high-dose intravenous
cyclophosphamide. Ann Rheum Dis. (2010) 69:61–4. doi: 10.1136/ard.2008 .1
02533
80. Zeher M, Doria A, L an J, Aroca G, Jayne D, Boletis I, et al. Efficacy
and safety of enteric-coated mycophenolate sodium in combination
with two glucocorticoid regimens for the treatment of active lupus
nephritis. Lupus. (2011) 20:1484–93. doi: 10.1177/09612033114
18269
81. Liu Z, Zhang H, Liu Z, Xing C, Fu P, Ni Z, et al. Multitarget therapy
for induction treatment of lupus nephritis. Ann Intern Med. (2015)
162:18. doi: 10.7326/M14-1030
82. Zhang H, Liu Z, Zhou M, Liu Z, Chen J, Xing C, et al. Multitarget therapy
for maintenance treatment of lupus nephritis. J Am Soc Nephrol. (2017)
28:3671–8. doi: 10.1681/ASN.2017030263
83. Ayoub I, Rovin BH. Calcineurin inhibitors in the treatment of lupus
nephritis: a hare versus turtle story? J Am Soc Nephrol. (2017) 28:3435–
7. doi: 10.1681/ASN.2017080830
84. Rovin BH, Solomons N, Pendergraft WF, Dooley MA, Tumlin J, Romero-
Diaz J, et al. A randomized, controlled double-blind study comparing the
efficacy and safety of dose-ranging voclosporin with placebo in achieving
remission in patients with active lupus nephritis. Kidney Int. (2019) 95:219–
31. doi: 10.1016/j.kint.2018.08.025
85. Appel GB, Contreras G, Dooley MA, Ginzler EM, Isenberg D, Jayne
D, et al. Mycophenolate mofetil versus cyclophosphamide for induction
treatment of lupus nephritis. J Am Soc Nephrol. (2009) 20:1103–
12. doi: 10.1681/ASN.2008101028
86. Condon MB, Ashby D, Pepper RJ, Cook HT, Levy JB, Griffith M, et
al. Prospective observational single-centre cohort study to evaluate
the effectiveness of treating lupus nephritis with rituximab and
mycophenolate mofetil but no oral steroids. Ann Rheum Dis. (2013)
72:1280–6. doi: 10.1136/annrheumdis-2012-202844
87. Roccatello D, Sciascia S, Baldovino S, Rossi D, Alpa M, Naretto C,
et al. A 4-year observation in lupus nephritis patients treated with
an intensified B-lymphocyte depletion without immunosuppressive
Frontiers in Medicine | www.frontiersin.org 10 February 2021 | Volume 8 | Article 622225

Mejía-Vilet and Ayoub The Use of Glucocorticoids in Lupus Nephritis
maintenance treatment—Clinical response compared to literature
and immunological re-assessment. Autoimmun Rev. (2015)
14:1123–30. doi: 10.1016/j.autrev.2015.07.017
88. Furie R, Rovin BH, Houssiau F, Malvar A, Teng YKO, Contreras G, et al.
Two-year, randomized, controlled trial of belimumab in lupus nephritis. N
Engl J Med. (2020) 383:1117–28. doi: 10.1056/NEJMoa2001180
89. Rovin BH, Aroca-Martinez G, Alvarez A, Fragoso-Loyo HE, Zut a AE,
Furie R, et al. Two-year results from a randomized, controlled study
of obinutuzumab for proliferative lupus nephritis. J Am Soc Nephrol.
(2020) 31:53.
90. Ruiz-Irastorza G, Ugarte A, Saint-Pastou Terrier C, Lazaro E, Iza A,
Couzi L, et al. Repeated pulses of methyl-prednisolone with reduced
doses of prednisone improve the outcome of class III, IV and V
lupus nephritis: an observational comparative study of the Lupus-
Cruces and lupus-Bordeaux cohorts. Autoimmun Rev. (2017) 16:826–
32. doi: 10.1016/j.autrev.2017.05.017
91. Edwards JCW, Snaith ML, Isenberg DA. A double blind controlled
trial of methylprednisolone infusions in systemic lupus erythematosus
using individualised outcome assessment. Ann Rheum Dis. (19 8 7) 46:773–
6. doi: 10.1136/ard.46.10.773
92. Pepper R, Griffith M, Kirwan C, Levy J, Taube D, Pusey C, et al.
Rituximab is an effective treatment for lu pus nephritis and allows a
reduction in maintenance steroids. Nephrol Dial Transplant. (2009) 24:3717–
23. doi: 10.1093/ndt/gfp336
93. Ezeonyeji AN, Isenberg DA. E arly treatment with rituximab in newly
diagnosed systemic lupus erythematosus patients: a steroid-sparing regimen.
Rheumatology. (2012) 51:476–81. doi: 10.1093/rheumatology/ker337
94. Rovin BH, Furie R, Latinis K, Looney RJ, Fervenza FC, Sanchez-Guerrero J, et
al. Efficacy and s afety of rituximab in patients with active proliferative lupus
nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis
Rheum. (2012) 64:1215–26. doi: 10.1002/art.34359
95. Gomez Mendez LM, Cascino MD, Garg J, Katsumoto TR, Brakeman P,
Dall’Era M, et al. Peripheral blood B cell depletion after rituximab and
complete response in lupus nephritis. Clin J Am Soc Nephrol. (2018) 13:1502–
9. doi: 10.2215/CJN.01070118
96. Sciascia S, Radin M, Yazdany J, Tektonidou M, Cecchi I, Roccatello
D, et al. Expanding t he therapeutic options for renal involvement in
lupus: eculizumab, available evidence. Rheumatol Int. (2017) 37:1249–
55. doi: 10.1007/s00296-017-3686-5
97. Wright RD, Bannerman F, Beresford MW, Oni L. A systematic
review of the role of eculizumab in systemic lupus erythematosus-
associated thrombotic microangiopathy. BMC Nephrol. (2020)
21:245 doi: 10.1186/s12882-020-01888-5
98. Jayne DRW, Bruchfeld AN, Harper L, Schaier M, Venning MC,
Hamilton P, et al. Randomized trial of C5 a receptor inhibitor avacopan
in ANCA-associated vasculitis. J Am Soc Nephrol. (2017) 28:275 6–
67. doi: 10.1681/ASN.2016111179
99. Jayne DR, Merkel PA, Yue H, Kelleher CL, Schall TJ, Bekker P. Complement
C5a receptor inhibitor avacopan improves renal function in ANCA
vasculitis. J Am Soc Nephrol. (2020) 31:53.
100. Kasitanon N, Fine DM, Haas M, Magder LS , Petri M. Hydroxychloroquine
use predicts complete renal remission within 12 months among patients
treated with mycophenolate mofetil therapy for membranous lupus
nephritis. Lupus. (2006) 15:366–70. doi: 10.1191/0961203306lu2313oa
101. Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA.
Clinical efficacy and side effects of antimalarials in systemic lupus
erythematosus: a systematic review. Ann Rheum Dis. (20 1 0) 69:2 0–
8. doi: 10.1136/ard.2008.101766
102. Mejia-Vilet JM, Cordova-Sanchez BM, Uribe-Uribe NO, Correa-
Rotter R. Immunosuppressive treatment for pure membranous
lupus nephropathy in a Hispanic population. Clin Rheumatol. (2016)
35:2219–27. doi: 10.1007/s10067-016-3366-y
103. Fessler BJ, Alarcón GS, McGwin G, Roseman J, Bastian HM, Friedman AW,
et al. Systemic lupus erythematosus in three ethnic groups: XVI. Association
of hydroxychloroquine use with reduced risk of damage accrual. Arthritis
Rheum. (2005) 52:1473–80. doi: 10.1002/art.21039
104. Pons-Estel GJ, Alarcón GS, McGwin G, Danila MI, Zhang J, Bastian HM, et
al. Protective effect of hydroxychloroquine on renal damage in patients with
lupus nephritis: LXV, data f rom a multiethnic US cohort. Arthritis Rheum.
(2009) 61:830–9. doi: 10.1002/art.24538
105. Shinjo SK, Bonfá E, Wojdyla D, Borba EF, Ramirez LA, Scherbarth HR, et al.
Antimalarial tre atment may have a time-dependent effect on lupus survival:
data from a multinational Latin American inception cohort. Arthritis Rheum.
(2010) 62:855–62. doi: 10.1002/art.27300
106. Mathian A, Pha M, Haroche J, Cohen-Aubart F, Hié M, Pineton de
Chambrun M, et al. Withdrawal of low-dose prednisone in SLE patients with
a clinically quiescent disease for more than 1 year: a randomised clinical
trial. Ann Rheum Dis. (2020) 79:339–46. doi: 10.1136/annrheumdis-2019-2
16303
107. Miloslavsky EM, Naden RP, Bijlsma JWJ, Brogan PA, Brown ES,
Brunetta P, et al. Development of a Glucocorticoid Toxicity Index (GTI)
using multicriteria decision analysis. Ann Rheum Dis. (2017) 76:543–
6. doi: 10.1136/annrheumdis-2016-210002
108. Goswami RP, Sit H, Ghosh P, Sircar G, Ghosh A. Steroid-free remission in
lupus: myth or reality; an observational study from a tertiary referral centre.
Clin Rheumatol. (2019) 38:1089–97. doi: 10.1007/s10067-0 18 -4 3 77 -7
109. Strehl C, Bijlsma JWJ, de Wit M, Boers M, Caeyers N, Cutolo M, et
al. Defining conditions where long-term glucocorticoid treatment has
an acceptably low level of harm to facilitate implementation of existing
recommendations: viewpoints from an EULAR task force. Ann Rheum Dis.
(2016) 75:952–7. doi: 10.1136/annrheumdis-2015-208916
110. Costedoat-Chalumeau N, Amoura Z, Hulot JS, Hammoud HA, Aymard G,
Cacoub P, et al. Low blood concentration of hydroxychloroquine is a marker
for and predictor of disease exacerbations in patients with systemic lupus
erythematosus. Arthritis Rheum. (2006) 54:3284–90. doi: 10.100 2/ art.2215 6
111. Costedoat-Chalumeau N, Galicier L, Aumaître O, Francès C, Guern V Le,
Lioté F, et al. Hydroxychloroquine in systemic lupus erythematosus: results of
a French multicentre controlled trial (PLUS Study). Ann Rheum Dis. (2013)
72:1786–92. doi: 10.1136/annrheumdis-2012-202322
112. Zen M, Saccon F, Gatto M, Montesso G, Larosa M, Benvenuti F, et al.
Prevalence and predictors of flare after immunosuppressant discontinuation
in patients with systemic lupus erythematosus in remission. Rheumatology.
(2020) 59:1591–8. doi: 10.1093/rheumatology/kez422
113. Moroni G, Gallelli B, Quaglini S, Banfi G, Rivolta E, Messa P,
et al. Wit hdrawal of therapy in patients with proliferative lupus
nephritis: long-term follow-up. Nephrol Dial Transplant. (2006) 21:1541–
8. doi: 10.1093/ndt/gfk073
114. Petri M, Purvey S, Fang H, Magder LS. Predictors of organ damage in
systemic lupus erythematosus: the Hopkins Lupus Cohort. Arthritis Rheum.
(2012) 64:4021–8. doi: 10.1002/art.34672
115. Camous L, Melander C, Vallet M, Squalli T, Knebelmann B, Noël LH, et
al. Complete remission of lupus nephritis with rituximab and steroids for
induction and rituximab alone for maintenance therapy. Am J Kidney Dis.
(2008) 52:346–52. doi: 10.1053/j.ajkd.2008.03.036
116. Sciascia S, Radin M, Yazdany J, Levy R, Roccatello D, Dall’Era M, et
al. Efficacy of belimumab on renal outcomes in patients with systemic
lupus erythematosus: a systematic review. Autoimmun Rev. (2017) 16:287–
93. doi: 10.1016/j.autrev.2017.01.010
117. Binda V, Tre z zi B, Del Papa N, Beretta L, Frontini G, Porata G, et al.
Belimumab may decrease flare rate and allow glucocorticoid withdrawal
in lupus nephritis (including dialysis and transplanted patient). J Nephrol.
(2020) 33:1019–25. doi: 10.1007/s40620-02 0 -0 07 06 - 3
118. Kraaij T, Huizinga TWJ, Rabelink TJ, Teng YKO. Belimumab after rituximab
as maintenance therapy in lupus nephritis. Rheumatology. (2014) 53:2122–
4. doi: 10.1093/rheumatology/keu369
Conflict of Interest: The authors declare that the research was conducted in the
absence of any commercial or financial relationships that could be construed as a
potential conflict of interest.
Copyright © 2021 Mejía-Vilet and Ayoub. This is an open-access article distributed
under the terms of the Creative Commons Attribution License (CC BY). The use,
distribution or reproduction in other forums is permitted, provided the original
author(s) and the copyright owner(s) are credited and that the original publication
in this journal is cited, in accordance with accepted academic practice. No use,
distribution or reproduction is permitted which does not comply with these terms.
Frontiers in Medicine | www.frontiersin.org 11 February 2021 | Volume 8 | Article 622225
